Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice - PubMed (original) (raw)
Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice
F Gouyon et al. J Physiol. 2003.
Abstract
The physiological significance of the presence of GLUT2 at the food-facing pole of intestinal cells is addressed by a study of fructose absorption in GLUT2-null and control mice submitted to different sugar diets. Confocal microscopy localization, protein and mRNA abundance, as well as tissue and membrane vesicle uptakes of fructose were assayed. GLUT2 was located in the basolateral membrane of mice fed a meal devoid of sugar or containing complex carbohydrates. In addition, the ingestion of a simple sugar meal promoted the massive recruitment of GLUT2 to the food-facing membrane. Fructose uptake in brush-border membrane vesicles from GLUT2-null mice was half that of wild-type mice and was similar to the cytochalasin B-insensitive component, i.e. GLUT5-mediated uptake. A 5 day consumption of sugar-rich diets increased fructose uptake fivefold in wild-type tissue rings when it only doubled in GLUT2-null tissue. GLUT5 was estimated to contribute to 100 % of total uptake in wild-type mice fed low-sugar diets, falling to 60 and 40 % with glucose and fructose diets respectively; the complement was ensured by GLUT2 activity. The results indicate that basal sugar uptake is mediated by the resident food-facing SGLT1 and GLUT5 transporters, whose mRNA abundances double in long-term dietary adaptation. We also observe that a large improvement of intestinal absorption is promoted by the transient recruitment of food-facing GLUT2, induced by the ingestion of a simple-sugar meal. Thus, GLUT2 and GLUT5 could exert complementary roles in adapting the absorption capacity of the intestine to occasional or repeated loads of dietary sugars.
Figures
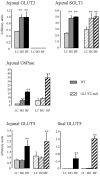
Figure 1. Transporter mRNA expression upon adaptation to carbohydrate diets
Intestinal RNA samples were extracted from mucosal scrapping of the upper jejunum or of the ileum of wild-type (WT, filled bars) or GLUT-2 null (hatched bars) mice. Messenger abundance was quantified either by density scanning of Northern blots (GLUT2, SGLT1, GLUT5) or by RT-PCR in real time with a Light-Cycler (GLUT2, glucose 6-phosphatase (G6Pase)). Results were obtained with an average 6 mice fed low carbohydrate (LC, light grey), glucose-rich (HG, grey) or fructose-rich (HF, dark grey) diets for 5 days. All quantifications were normalized against L19 or the 18S ribosomal RNA. Results are expressed in arbitrary units ±
s.e.m.
; ** significant difference (P < 0.01) of data when compared with LC diet.
Figure 2. GLUT2 protein expression in purified brush-border membrane
Intestinal brush-border membranes (BBM, 40 μg) were prepared from the jejunum of wild-type (WT) or GLUT2-null (Null) mice. Western blots were then performed as described in Methods. ECL-films were exposed for an average 3 min. A, presence of GLUT2 in purified BBM preparation. The purity of BBM vesicles is shown. E-cadherin, a basolateral membrane marker, is present only in crude membrane fractions (CM) and absent in corresponding purified BBM. SGLT-1 and PepT-1 brush membrane markers are enriched in the BBM fraction. B, presence of GLUT2 in BBM from wild-type and absence from GLUT2-null mice fed a glucose-rich or a fructose-rich diet. C, expression of GLUT2 in BBM preparations from wild-type mice submitted to dietary manipulations. GLUT2 was absent from the BBM of mice receiving a PBS bolus after an overnight fast or mice fed a standard chow ad libitum. GLUT2 was present in the BBM of mice fed a fructose diet ad libitum, or in BBM of mice that had received a 40 % glucose, fructose, glucose + fructose or sucrose gastric bolus after an overnight fast. Experiments represent 2–3 independent BBM preparations with 6–9 mice.
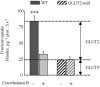
Figure 3. Fructose uptake in jejunal BBM vesicles
The uptake of 10 m
m
fructose was measured in isolated BBM vesicles at 35°C for 5 s in the presence or absence of 10 μ
m
cytochalasin B. Wild-type and GLUT2-null mice were fed a fructose diet for 5 days, and 30 min after a fructose bolus the intestine was dissected. We used 3 membrane-vesicle preparations from 3 independent experiments with 5 mice per membrane preparation. Results are expressed as picomoles per microgram of protein per 5 seconds ±
s.e.m.
, (n = 6–10 determinations; ***P < 0.001). Data were corrected for non-carrier mediated diffusion as estimated by 10 m
ml
-glucose uptakes, measured in parallel.
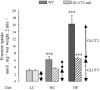
Figure 4. Fructose uptake in jejunal rings
Wild-type (filled bars) and GLUT2-null (hatched bars) mice were fed a low carbohydrate (LC, light grey), glucose-rich (HG, medium grey) or fructose-rich (HF, dark grey) diet for 5 days. Everted intestinal rings were incubated for 2 min in 50 m
m
fructose. Results are expressed as nanomoles per milligram wet weight per 2 minutes ±
s.e.m.
obtained in 3 separate experiments with at least 15 uptake determinations. Each condition has been performed with at least 12 mice per group. Statistical significance, ***P < 0.001.
Figure 5. Localization of GLUT2 by immunohistochemistry in the membrane domain of enterocytes
Mice were fed either the low carbohydrate (sugar deprived, B) or the fructose-rich diet together a fructose load (A, C, D, E). Antibody against the extracellular loop of GLUT2 (green) was used in GLUT2-null (A) and wild-type (B-E) mice. The TOTO-3 label of nuclei is in blue (B and C). A cross-section of a villus from a sugar-deprived animal is shown (B). Note the fluorescence of GLUT2 in the basolateral membrane but not in the apical membrane domains of the cells. GLUT2 is present in the apical and basolateral membranes of fructose-loaded mice (C). A higher magnification of an intestinal villus lining three enterocytes in fructose-stimulated mice is shown (D). Figures are representative of at least three immunolabelling experiments. Scale (white bars) 20 μm. Note the similarity between GLUT2-null background (A) and that obtained with peptide-blocked GLUT2 antibodies (E). Arrowheads point to the apical membrane domain of enterocytes.
Similar articles
- Sugar sensing by enterocytes combines polarity, membrane bound detectors and sugar metabolism.
Le Gall M, Tobin V, Stolarczyk E, Dalet V, Leturque A, Brot-Laroche E. Le Gall M, et al. J Cell Physiol. 2007 Dec;213(3):834-43. doi: 10.1002/jcp.21245. J Cell Physiol. 2007. PMID: 17786952 - Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice.
Tobin V, Le Gall M, Fioramonti X, Stolarczyk E, Blazquez AG, Klein C, Prigent M, Serradas P, Cuif MH, Magnan C, Leturque A, Brot-Laroche E. Tobin V, et al. Diabetes. 2008 Mar;57(3):555-62. doi: 10.2337/db07-0928. Epub 2007 Dec 5. Diabetes. 2008. PMID: 18057092 - GLUT2 mutations, translocation, and receptor function in diet sugar managing.
Leturque A, Brot-Laroche E, Le Gall M. Leturque A, et al. Am J Physiol Endocrinol Metab. 2009 May;296(5):E985-92. doi: 10.1152/ajpendo.00004.2009. Epub 2009 Feb 17. Am J Physiol Endocrinol Metab. 2009. PMID: 19223655 Review. - Intestinal fructose transport and malabsorption in humans.
Jones HF, Butler RN, Brooks DA. Jones HF, et al. Am J Physiol Gastrointest Liver Physiol. 2011 Feb;300(2):G202-6. doi: 10.1152/ajpgi.00457.2010. Epub 2010 Dec 9. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21148401 Review.
Cited by
- GLUT5 (SLC2A5) enables fructose-mediated proliferation independent of ketohexokinase.
Liang RJ, Taylor S, Nahiyaan N, Song J, Murphy CJ, Dantas E, Cheng S, Hsu TW, Ramsamooj S, Grover R, Hwang SK, Ngo B, Cantley LC, Rhee KY, Goncalves MD. Liang RJ, et al. Cancer Metab. 2021 Mar 24;9(1):12. doi: 10.1186/s40170-021-00246-9. Cancer Metab. 2021. PMID: 33762003 Free PMC article. - Stress and glucocorticoid inhibit apical GLUT2-trafficking and intestinal glucose absorption in rat small intestine.
Shepherd EJ, Helliwell PA, Mace OJ, Morgan EL, Patel N, Kellett GL. Shepherd EJ, et al. J Physiol. 2004 Oct 1;560(Pt 1):281-90. doi: 10.1113/jphysiol.2004.072447. Epub 2004 Aug 5. J Physiol. 2004. PMID: 15297580 Free PMC article. - Fructose reabsorption by rat proximal tubules: role of Na+-linked cotransporters and the effect of dietary fructose.
Gonzalez-Vicente A, Cabral PD, Hong NJ, Asirwatham J, Saez F, Garvin JL. Gonzalez-Vicente A, et al. Am J Physiol Renal Physiol. 2019 Mar 1;316(3):F473-F480. doi: 10.1152/ajprenal.00247.2018. Epub 2018 Dec 19. Am J Physiol Renal Physiol. 2019. PMID: 30565998 Free PMC article. - Fructose metabolism in the cerebellum.
Funari VA, Crandall JE, Tolan DR. Funari VA, et al. Cerebellum. 2007;6(2):130-40. doi: 10.1080/14734220601064759. Cerebellum. 2007. PMID: 17510913 Review. - Dietary fructose, salt absorption and hypertension in metabolic syndrome: towards a new paradigm.
Soleimani M. Soleimani M. Acta Physiol (Oxf). 2011 Jan;201(1):55-62. doi: 10.1111/j.1748-1716.2010.02167.x. Acta Physiol (Oxf). 2011. PMID: 21143427 Free PMC article. Review.
References
- Bismut H, Hers HG, Van Schaftingen E. Conversion of fructose to glucose in the rabbit small intestine. A reappraisal of the direct pathway. Eur J Biochem. 1993;213:721–726. - PubMed
- Brot-Laroche E, Serrano MA, Delhomme B, Alvarado F. Different temperature sensitivity and cation specificity of two distinct D-glucose/Na+ cotransport systems in the intestinal brush-border membrane. Ann N Y Acad Sci. 1985;456:47–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous