Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors - PubMed (original) (raw)
Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors
Holger K Eltzschig et al. J Exp Med. 2003.
Abstract
Limited oxygen delivery to tissues (hypoxia) is common in a variety of disease states. A number of parallels exist between hypoxia and acute inflammation, including the observation that both influence vascular permeability. As such, we compared the functional influence of activated polymorphonuclear leukocytes (PMN) on normoxic and posthypoxic endothelial cells. Initial studies indicated that activated PMN preferentially promote endothelial barrier function in posthypoxic endothelial cells (>60% increase over normoxia). Extension of these findings identified at least one soluble mediator as extracellular adenosine triphosphate (ATP). Subsequent studies revealed that ATP is coordinately hydrolyzed to adenosine at the endothelial cell surface by hypoxia-induced CD39 and CD73 (>20-and >12-fold increase in mRNA, respectively). Studies in vitro and in cd39-null mice identified these surface ecto-enzymes as critical control points for posthypoxia-associated protection of vascular permeability. Furthermore, insight gained through microarray analysis revealed that the adenosine A2B receptor (AdoRA2B) is selectively up-regulated by hypoxia (>5-fold increase in mRNA), and that AdoRA2B antagonists effectively neutralize ATP-mediated changes in posthypoxic endothelial permeability. Taken together, these results demonstrate transcription coordination of adenine nucleotide and nucleoside signaling at the vascular interface during hypoxia.
Figures
Figure 1.
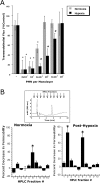
Influence of PMN on permeability of normoxic and posthypoxic endothelia. (A) Activated PMN (10−6 M FMLP) at indicated concentrations were added to the apical surface of confluent normoxic (48 h exposure to pO2 147) or posthypoxic (48 h exposure to pO2 20 torr) HMEC-1 and permeability to FITC-dextran (70 kD) was quantified. Transendothelial flux was calculated by linear regression (4 samples over 60 min) and normalized as percent of control (HBSS). Data are derived from six monolayers in each condition. Data are expressed as mean ± SD of percent control flux with HBSS only. Asterisk (*) indicates significant differences from baseline (P < 0.05) and a cross (#) indicates differences from baseline and from normoxia (P < 0.05). (B) Supernatants derived from activated PMN (10−6 M FMLP for 10 min) were fractionated by HPLC (H20 mobile phase at 1 ml/min, 1 ml samples; inset indicates representative UV spectra at 260 nm and indicated samples collected at individual arrows). Samples were concentrated 20-fold and tested for bioactivity on normoxic or posthypoxic HMEC-1 in a model of paracellular permeability. Fraction #6 promotes barrier function in normoxic and posthypoxic endothelial cells, whereas fraction #2 is relatively selective for posthypoxic endothelia (*, P < 0.01 compared with HBSS).
Figure 2.
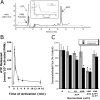
Activated PMN rapidly release ATP. (A) Chromatographic identification of ATP in supernatants derived from activated PMN. Shown here is a representative overlay chromatogram of activated PMN supernatant (bold line) and authentic ATP (narrow line). Inset represents an overlay UV spectra derived from fraction #2 (see Fig. 1) and of authentic ATP with one dominant peak at 260 nm. (B) Time course of ATP release from activated PMN (107 PMN/ml activated with 10−6 M FMLP). PMN were activated for indicated periods of time and supernatant ATP was quantified by luciferase assay. Data represent mean ± SD ATP/107 PMN for three separate experiments. (C) Authentic ATP selectively promotes endothelial barrier function in posthypoxic endothelia. Indicated concentrations of ATP were added to HMEC-1 preexposed to normoxia or hypoxia. As indicated, ATP influenced endothelial permeability only in posthypoxic endothelial cells (*, P < 0.025 compared with no ATP). In contrast, the addition of adenosine (Ado, 100 μM) was associated with an increase in barrier function in both normoxic and posthypoxic endothelial cells (#, P < 0.01 compared with buffer alone). The addition of 8-phenyl-theophylline (8-PT at 10 μM, a nonselective adenosine receptor antagonist) obviated the barrier effect of 100 μM ATP in posthypoxic endothelial cells. Data are derived from six monolayers in each condition. Data are expressed as mean ± SD of percent control flux with HBSS only.
Figure 3.
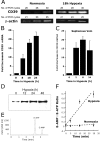
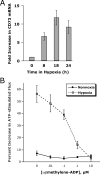
Induction of functional CD39 is by hypoxia. (A) Confluent HMEC-1 monolayers were exposed to normoxia (pO2 147 torr, 18 h) or hypoxia (pO2 20 torr, 18 h). Total RNA was isolated, and CD39 mRNA levels were determined by RT-PCR using semiquantitative analysis (increasing cycle numbers, as indicated). As shown, β-actin transcript was determined in parallel and used as a control. (B) Real-time PCR was employed to confirm hypoxia inducibility of CD39 in cultured endothelial cells (HMEC-1). Data were calculated relative to internal housekeeping gene (β-actin) and are expressed as fold increase over normoxia ± SD at each indicated time. Results are derived from three experiments in each condition. (C) Human saphenous vein was obtained from patients undergoing aorto-coronary bypass surgery and exposed ex vivo to ambient normoxia (pO2 147 torr, 24 h) or hypoxia (pO2 20 torr for 2, 8, or 24 h). After total RNA isolation, real-time PCR was performed to investigate CD39 inducibility by hypoxia. Data were calculated relative to internal control (β-actin) and are expressed as fold increase over normoxia ± SD at each indicated time. Results are derived from three experiments in each condition. (D) Increase in surface CD39 surface protein with hypoxic exposure. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia, monolayers were washed, surface proteins were biotinylated, and cells were lysed. CD39 was immunoprecipitated with mAb directed against human CD39. Immunoprecipitates were resolved by SDS-PAGE, and resultant Western blots were probed with avidin-peroxidase. A representative experiment of three is shown. (E) Validation etheno-ATP (E-ATP) and etheno-AMP (E-AMP) resolution by HPLC. Shown is a representative tracing indicating resolution of definitive peaks at UV 260 nm. (F) Functional increase in CD39 surface activity by hypoxia. Endothelial monolayers were exposed to 48 h hypoxia or normoxia, washed, and surface CD39 activity was determined by HPLC analysis of E-ATP conversion to E-AMP in the presence of the CD73-inhibitor αβ-methylene-ADP (10 μM, to prevent further metabolism of E-AMP to E-adenosine). Data are derived from five to seven monolayers in each condition, and results are expressed as E-AMP: E-ATP ratio ± SD.
Figure 4.
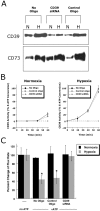
Role of CD39 in ATP-elicited changes of posthypoxic endothelial permeability. (A) HMEC-1 were loaded with CD39-specific siRNA, control ribonucleotide or mock treated (control) and exposed to hypoxia or normoxia (48 h). Monolayers were washed, surface protein was biotinylated, and cells were lysed. CD39 was immunoprecipitated and resolved by SDS-PAGE, and resultant Western blots were probed with avidin-peroxidase. As a control for specificity, CD73 protein induction by hypoxia was assessed in parallel. (B) Influence of CD39 suppression by siRNA on functional surface protein. HMEC-1 were loaded with CD39-specific siRNA, control-ribonucleotide, or mock treated and CD39 activity was determined by HPLC analysis of E-ATP conversion to E-AMP (in the presence of the CD73-inhibitor αβ-methylene-ADP). Data are derived from five to seven monolayers in each condition, and results are expressed as E-AMP: E-ATP ratio ± SD. (C) Influence of CD39 suppression by siRNA on endothelial barrier. HMEC-1 were loaded with CD39-specific siRNA, control ribonucleotide or mock treated (control), exposed to hypoxia or normoxia (48 h), and permeability to 70 kD FITC in the presence or absence of ATP (100 μM) was assessed (*, P < 0.01 compared with no ATP). Data are derived from six monolayers in each condition, and data are expressed as mean ± SD of percent control flux.
Figure 5.
Induction of CD73 by hypoxia is necessary for ATP-elicited increases in posthypoxic endothelial barrier function. (A) Induction of endothelial CD73 by hypoxia. Real-time PCR was employed to confirm hypoxia inducibility of CD73 in cultured endothelial cells (HMEC-1). Data were calculated relative to internal control genes (β-actin) and are expressed as fold increase over normoxia ± SD at each indicated time. Results are derived from three experiments in each condition. (B) Influence of CD73-inhibitor αβ-methylene-ADP on ATP-elicited changes of endothelial permeability. Indicated concentrations of αβ-methylene-ADP were added to HMEC-1 monolayers that were cultured under normoxic or hypoxic conditions (48 h) and stimulated with 100 μM ATP. In normoxic HMEC-1, neither the addition of ATP, nor αβ-methylene-ADP were associated with changes in paracellular flux. In contrast, ATP-elicited changes in endothelial flux in posthypoxic HMEC-1 were obviated in a concentration-dependent fashion (P < 0.01 by ANOVA). Data are derived from six monolayers in each condition. Data are expressed as mean ± SD of percent control flux with HBSS only.
Figure 6.
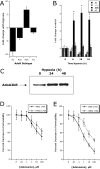
Adenosine A2B receptor (AdoRA2B) induction by hypoxia enhances barrier response. (A) Microarray analysis of individual adenosine receptors in response to hypoxia (AdoRA1, AdoRA2A, AdoRA2B, and AdoRA3). Confluent HMEC-1 were exposed to normoxia or hypoxia (12 h exposure to pO2 20 torr) and the relative expression of individual adenosine receptors was quantified from total RNA by microarray analysis. Data are expressed as fold change ± SD relative to normoxia. (B) Real-time PCR analysis was employed to confirm hypoxia-regulated expression of individual adenosine receptors (AdoRA1, AdoRA2A, AdoRA2B, and AdoRA3) in HMEC-1. Data were calculated relative to internal control (β-actin) and are expressed as fold increase ± SD over normoxia at indicated time points. Results are derived from three experiments in each condition (*, P < 0.01 compared with normoxia). (C) Increased AdoRA2B surface protein with hypoxia. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia, monolayers were washed, surface proteins were biotinylated, and cells were lysed. AdoRA2B was immunoprecipitated with mAb to human AdoRA2B and resolved by SDS-PAGE, and resultant Western blots were probed with avidin-peroxidase. A representative experiment of three is shown. (D) Hypoxia induction of AdoRA2B enhances barrier response of HMEC-1 to adenosine. Indicated concentrations of adenosine were added to HMEC-1 monolayers preexposed to normoxia or hypoxia (48 h). Addition of the specific AdoRA2B-antagonist MRS 1754 (100 nM) significantly shifted the adenosine dose response in posthypoxic endothelium (P < 0.01 by ANOVA). Data are derived from 6 monolayers in each condition. Data are expressed as mean ± SD of percent control flux.
Figure 7.
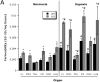
Role of hypoxia-induced CD39 in vivo: cd39-deficient mice and age, weight, and gender matched controls were administered intravenous Evans blue solution (0.2 ml of 0.5% in PBS) and exposed to normobaric hypoxia (8% O2, 92% N2) or room air for 4 h. Animals were killed and the colon, muscle, kidney, brain, liver, and lungs were harvested. Organ Evans blue concentrations were quantified following formamide extraction (55°C for 2 h) by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. _cd39_-deficient mice showed higher tissue Evans-blue concentrations in all organs (except muscle) in normoxic and hypoxic conditions as compared with wild-type animals (*, P < 0.05). Hypoxic wild-type and _cd39_-deficient mice showed higher tissue Evans blue concentrations than normoxic mice, with the exception of hypoxic colon of the _cd39_-deficient mice (#, P < 0.05). Data are expressed as mean ± SD Evans blue OD/mg wet tissue, and are pooled from four to six animals per condition. Images of abdominal dissections (B) and isolated colons (C) from wild-type and _cd39_-deficient mice subjected to normoxia and hypoxia. Note increased Evans blue retention with hypoxia and marked differences between wild-type and _cd39_-deficient animals under both conditions.
Figure 7.
Role of hypoxia-induced CD39 in vivo: cd39-deficient mice and age, weight, and gender matched controls were administered intravenous Evans blue solution (0.2 ml of 0.5% in PBS) and exposed to normobaric hypoxia (8% O2, 92% N2) or room air for 4 h. Animals were killed and the colon, muscle, kidney, brain, liver, and lungs were harvested. Organ Evans blue concentrations were quantified following formamide extraction (55°C for 2 h) by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. _cd39_-deficient mice showed higher tissue Evans-blue concentrations in all organs (except muscle) in normoxic and hypoxic conditions as compared with wild-type animals (*, P < 0.05). Hypoxic wild-type and _cd39_-deficient mice showed higher tissue Evans blue concentrations than normoxic mice, with the exception of hypoxic colon of the _cd39_-deficient mice (#, P < 0.05). Data are expressed as mean ± SD Evans blue OD/mg wet tissue, and are pooled from four to six animals per condition. Images of abdominal dissections (B) and isolated colons (C) from wild-type and _cd39_-deficient mice subjected to normoxia and hypoxia. Note increased Evans blue retention with hypoxia and marked differences between wild-type and _cd39_-deficient animals under both conditions.
Figure 8.
Proposed model of coordinated nucleotide metabolism and nucleoside signaling in posthypoxic endothelial cells: in areas of ongoing inflammation, diminished oxygen supply coordinates the induction of CD39, CD73, and AdoRA2B. At such sites, activated PMN provide a readily available extracellular source of ATP that through two enzymatic steps results in the liberation of extracellular adenosine. Adenosine generated in this fashion is available for activation of surface endothelial adenosine receptors, particularly the AdoRA2B. Postreceptor increases in intracellular cyclic AMP results in enhanced barrier function. As such, this protective mechanism may provide an innate mechanism to preserve vascular integrity and prevent fulminant intravascular fluid loss.
Similar articles
- Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism.
Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Eltzschig HK, et al. Blood. 2004 Dec 15;104(13):3986-92. doi: 10.1182/blood-2004-06-2066. Epub 2004 Aug 19. Blood. 2004. PMID: 15319286 - ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function.
Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP. Eltzschig HK, et al. Circ Res. 2006 Nov 10;99(10):1100-8. doi: 10.1161/01.RES.0000250174.31269.70. Epub 2006 Oct 12. Circ Res. 2006. PMID: 17038639 - Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface.
Eltzschig HK, Macmanus CF, Colgan SP. Eltzschig HK, et al. Trends Cardiovasc Med. 2008 Apr;18(3):103-7. doi: 10.1016/j.tcm.2008.01.006. Trends Cardiovasc Med. 2008. PMID: 18436149 Free PMC article. Review. - Neutrophil-derived 5'-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation.
Lennon PF, Taylor CT, Stahl GL, Colgan SP. Lennon PF, et al. J Exp Med. 1998 Oct 19;188(8):1433-43. doi: 10.1084/jem.188.8.1433. J Exp Med. 1998. PMID: 9782120 Free PMC article. - Nucleotide metabolism and cell-cell interactions.
Eltzschig HK, Weissmüller T, Mager A, Eckle T. Eltzschig HK, et al. Methods Mol Biol. 2006;341:73-87. doi: 10.1385/1-59745-113-4:73. Methods Mol Biol. 2006. PMID: 16799190 Review.
Cited by
- Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury.
Koeppen M, McNamee EN, Brodsky KS, Aherne CM, Faigle M, Downey GP, Colgan SP, Evans CM, Schwartz DA, Eltzschig HK. Koeppen M, et al. Mucosal Immunol. 2013 Jul;6(4):762-75. doi: 10.1038/mi.2012.114. Epub 2012 Nov 28. Mucosal Immunol. 2013. PMID: 23187315 Free PMC article. - Decreased extracellular adenosine levels lead to loss of hypoxia-induced neuroprotection after repeated episodes of exposure to hypoxia.
Cui M, Bai X, Li T, Chen F, Dong Q, Zhao Y, Liu X. Cui M, et al. PLoS One. 2013;8(2):e57065. doi: 10.1371/journal.pone.0057065. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437309 Free PMC article. - Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia.
Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Eckle T, et al. Nat Med. 2012 Apr 15;18(5):774-82. doi: 10.1038/nm.2728. Nat Med. 2012. PMID: 22504483 Free PMC article. - Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa.
Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK. Clambey ET, et al. Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):E2784-93. doi: 10.1073/pnas.1202366109. Epub 2012 Sep 17. Proc Natl Acad Sci U S A. 2012. PMID: 22988108 Free PMC article. - Tissue-resident ecto-5' nucleotidase (CD73) regulates leukocyte trafficking in the ischemic brain.
Petrovic-Djergovic D, Hyman MC, Ray JJ, Bouis D, Visovatti SH, Hayasaki T, Pinsky DJ. Petrovic-Djergovic D, et al. J Immunol. 2012 Mar 1;188(5):2387-98. doi: 10.4049/jimmunol.1003671. Epub 2012 Jan 30. J Immunol. 2012. PMID: 22291183 Free PMC article.
References
- Tamura, D.Y., E.E. Moore, D.A. Partrick, J.L. Johnson, P.J. Offner, and C.C. Silliman. 2002. Acute hypoxemia in humans enhances the neutrophil inflammatory response. Shock. 17:269–273. - PubMed
- Collard, C.D., K.A. Park, M.C. Montalto, S. Alapati, J.A. Buras, G.L. Stahl, and S.P. Colgan. 2002. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J. Biol. Chem. 277:14801–14811. - PubMed
- Rui, T., G. Cepinskas, Q. Feng, Y.S. Ho, and P.R. Kvietys. 2001. Cardiac myocytes exposed to anoxia-reoxygenation promote neutrophil transendothelial migration. Am. J. Physiol. Heart Circ. Physiol. 281:H440–H447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R29 DK050189/DK/NIDDK NIH HHS/United States
- R37 DK050189/DK/NIDDK NIH HHS/United States
- DK 50189/DK/NIDDK NIH HHS/United States
- HL 60569/HL/NHLBI NIH HHS/United States
- R01 HL060569/HL/NHLBI NIH HHS/United States
- P01 DE013499/DE/NIDCR NIH HHS/United States
- DE 13499/DE/NIDCR NIH HHS/United States
- Z01 DK031116-20/Intramural NIH HHS/United States
- R01 DK050189/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials