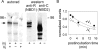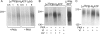Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating - PubMed (original) (raw)
Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating
Claudia Basso et al. J Gen Physiol. 2003 Sep.
Abstract
CFTR, the protein defective in cystic fibrosis, functions as a Cl- channel regulated by cAMP-dependent protein kinase (PKA). CFTR is also an ATPase, comprising two nucleotide-binding domains (NBDs) thought to bind and hydrolyze ATP. In hydrolyzable nucleoside triphosphates, PKA-phosphorylated CFTR channels open into bursts, lasting on the order of a second, from closed (interburst) intervals of a second or more. To investigate nucleotide interactions underlying channel gating, we examined photolabeling by [alpha32P]8-N3ATP or [gamma32P]8-N3ATP of intact CFTR channels expressed in HEK293T cells or Xenopus oocytes. We also exploited split CFTR channels to distinguish photolabeling at NBD1 from that at NBD2. To examine simple binding of nucleotide in the absence of hydrolysis and gating reactions, we photolabeled after incubation at 0 degrees C with no washing. Nucleotide interactions under gating conditions were probed by photolabeling after incubation at 30 degrees C, with extensive washing, also at 30 degrees C. Phosphorylation of CFTR by PKA only slightly influenced photolabeling after either protocol. Strikingly, at 30 degrees C nucleotide remained tightly bound at NBD1 for many minutes, in the form of nonhydrolyzed nucleoside triphosphate. As nucleotide-dependent gating of CFTR channels occurred on the time scale of seconds under comparable conditions, this suggests that the nucleotide interactions, including hydrolysis, that time CFTR channel opening and closing occur predominantly at NBD2. Vanadate also appeared to act at NBD2, presumably interrupting its hydrolytic cycle, and markedly delayed termination of channel open bursts. Vanadate somewhat increased the magnitude, but did not alter the rate, of the slow loss of nucleotide tightly bound at NBD1. Kinetic analysis of channel gating in Mg8-N3ATP or MgATP reveals that the rate-limiting step for CFTR channel opening at saturating [nucleotide] follows nucleotide binding to both NBDs. We propose that ATP remains tightly bound or occluded at CFTR's NBD1 for long periods, that binding of ATP at NBD2 leads to channel opening wherupon its hydrolysis prompts channel closing, and that phosphorylation acts like an automobile clutch that engages the NBD events to drive gating of the transmembrane ion pore.
Figures
Figure 1.
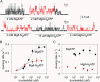
The 8-azido group slows CFTR Cl− channel opening and closing. (A) Outward currents (at +40 mV) activated by nucleotides indicated beneath traces in patches excised from oocytes expressing WT CFTR, after prior exposure to PKA plus 5 mM MgATP. (B and C) Mean rates of channel opening to a burst (rCO, B) and of closing from a burst (rOC, C) vs. [MgATP] (filled circles) or [Mg8-N3ATP] (red triangles), from analysis of records as in A (lower trace). For each test concentration (e.g., 15 μM Mg8-N3ATP, in A), kinetic parameters were normalized to the average of the values obtained from the same channels during the bracketing exposures to saturating nucleotide (e.g., 2 mM Mg8-N3ATP, in A). These ratios were then scaled by our estimates of absolute opening and closing rates at saturating [nucleotide] (see
materials and methods
): for MgATP, rCO(5 mM MgATP) = 0.27 ± 0.03 s−1 and rOC(5 mM MgATP)= 3.3 ± 0.2 s−1 (n = 18; Vergani et al., 2003). For Mg8-N3ATP we multiplied those rates by the factors (0.42 for rCO, and 0.19 for rOC) determined by direct comparison (as in A, top trace), assuming that 2 and 5 mM MgATP are both saturating concentrations. Curves in B show Michelis-Menten fits, with parameters (mean ± SEM; 2 ≤ n ≤ 7) K0.5 = 55 ± 5 μM and 11 ± 3 μM and Vmax = 1.02 ± 0.02 and 1.01 ± 0.04, for MgATP and Mg8-N3ATP, respectively. The high apparent affinity for Mg8-N3ATP is directly evident in Fig. 1 A, bottom trace: thus, rCO at 15 μM Mg8-N3ATP averaged 60 ± 11% (n = 7) of rCO at saturating, 2 mM, Mg8-N3ATP, whereas rCO at 15 μM MgATP was only 17 ± 1% (n = 8) of rCO at saturating [MgATP] (Fig. 1 B).
Figure 2.
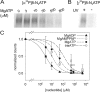
[α32P]8-N3ATP and [γ32P]8-N3ATP binding to Flag-CFTR in HEK293T cell membranes and inhibition by nucleotides. (A) Autoradiogram showing competition by cold MgATP for binding of 5 μM [α32P]8-N3ATP to CFTR at 0°C, followed by UV irradiation, washing, immunoprecipitation, and SDS-PAGE. (B) CFTR incubated with 5 μM [γ32P]8-N3ATP at 0°C was photolabeled only after UV irradiation, indicating lack of phosphorylation by endogenous kinases under these conditions. (C) Quantification of competition for binding of 5 μM [α32P]8-N3ATP by unlabeled MgATP (as in A), MgADP, or MgAMPPNP (all in Mg2+-containing buffer), or by unlabeled TrisATP (in Mg2+-free buffer). Photolabeling was normalized to the signal obtained in the absence of competing nucleotide (note broken abscissa), and the data (error bars show ±SD) fitted assuming simple competition and a Kd of 5 μM for Mg8-N3ATP, or 8 μM for free 8-N3ATP. Ki values from the fits were 1 ± 1 μM for MgADP (filled triangles, n = 2), 6 ± 2 μM for MgAMPPNP (empty squares, n = 4), 16 ± 4 μM for MgATP (filled circles, n = 4), and 69 ± 20 μM for TrisATP (empty circles, n = 3). Given the finite duration of UV irradiation, these apparent binding affinities might be less accurate than the ratios of the values obtained for the different nucleotides.
Figure 3.
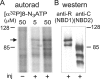
Binding of [α32P]8-N3ATP to split CFTR channels. (A) Membranes from oocytes coexpressing Flag-3–835 CFTR and Flag-837–1480 CFTR (+), or noninjected controls (−), incubated with 5 or 50 μM [α32P]8-N3ATP at 0°C were irradiated with UV before washing, immunoprecipitation, and SDS-PAGE. Arrow indicates photolabeled band in the autoradiogram corresponding to CFTR half containing NBD1. (B) Immunoblots of proteins immunoprecipitated and run in same SDS-PAGE gel used for A, before transfer to nitrocellulose and blotting with anti-R domain antibody (left) that recognizes Flag-3–835-containing NBD1, or with anti–COOH-terminal antibody (right) that recognizes Flag-837–1480-containing NBD2.
Figure 4.
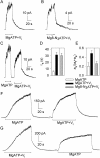
Vi locks open WT CFTR channels activated by PKA and either MgATP or Mg8-N3ATP. (A–C) Macroscopic current, in patches containing hundreds of channels excised from oocytes expressing WT CFTR, relaxed with double exponential time course (smooth fit lines, largely obscured by data) after removal of nucleotides. Nucleotides (0.5 mM in A and B; 5 mM in C) and Vi were applied in the presence of 300 nM PKA. Fit parameters for trace in A are, af = 25 pA, as = 24 pA, τf = 0.5 s, τs = 20 s; for trace in B, af = 14 pA, as = 7 pA, τf = 3 s, τs = 35 s; for first decay in C, af = 50 pA, τf = 0.5 s; second decay in C, af = 27 pA, as = 18 pA, τf = 0.6 s, τs = 21 s. Solution exchange took <1 s, estimated from current decay after brief activation of endogenous Ca2+-activated Cl− channels. (D and E) Summary of mean (±SEM, n = 6) time constants (D) and fractional amplitudes (E) of slow components of current decay after activation by PKA and 0.5 mM MgATP without (white bars), or with (black bars) 5 mM Vi, or by PKA and 0.5 mM Mg8-N3ATP with 5 mM Vi (gray bars). (F and G) Macroscopic current records from inside-out patches excised from HEK293T cells expressing WT CFTR; MgATP (5 mM) and Vi (5 mM) applications were in the presence of 300 nM PKA; the breaks indicate omission of 30 s (F) and 100 s (G) of record. (In C, F, and G, offset bars beneath traces signal different solution compositions.)
Figure 5.
(A) In split CFTR, tightly bound [α32P]8-azido nucleotide predominantly labels NBD1. (Left) Autoradiogram from membranes of oocytes coexpressing Flag-3–835 and Flag-837–1480 (+), or of noninjected oocytes (−) incubated for 15 min at 30°C with 5 μM [α32P]8-N3ATP and 10 mM Mg2+ ± 1 mM Vi, subjected to the standard wash procedure including 5-min postincubation at 30°C, irradiated with UV, immunoprecipitated, and subjected to SDS-PAGE. Arrow indicates photolabeled band corresponding to CFTR half containing NBD1. (Right) Proteins from the same membranes were immunoprecipitated and blotted with either anti–R-domain antibody, which recognizes the half of CFTR containing NBD1 (left two lanes), or with anti–COOH-terminal antibody, which recognizes the half containing NBD2 (right two lanes). (B) Extremely slow release of [α32P]8-azido nucleotide during nucleotide-free postincubation at 30°C before UV irradiation. Initial incubation of membranes from HEK293T cells expressing Flag-CFTR was with 5 μM [α32P]8-N3ATP for 15 min at 30°C with (+Vi) or without (−Vi) 1 mM Vi. After postincubation for time indicated, immunoprecipitation, and SDS-PAGE, the resulting autoradiogram signals were normalized to that obtained with Vi but without postincubation (postincubation time = 0) in each experiment (n = 6 for 0- and 5-min points; n = 2 for 1.5- and 15-min points; error bars give ± SD). Curves show simultaneous least-squares fits to both datasets with single time constant, yielding τ = 15 ± 2 min.
Figure 6.
Tightly bound 8-N3 nucleotide at NBD1 is largely ATP. (A) Autoradiogram showing labeling of CFTR by [γ32P]8-N3ATP without UV (reflecting protein phosphorylation; right) and after UV irradiation (reflecting both photocrosslinking and phosphorylation; left). Prephosphorylated (with PKA; see Fig. 7) HEK293T membranes containing Flag-CFTR were incubated for 15 min at 30°C with 5 μM [γ32P]8-N3ATP plus 1 mM Vi, subjected to usual washes plus 5-min nucleotide-free postincubation at 30°C, exposed to UV (+) or kept in the dark (−), immunoprecipitated, run on SDS-PAGE, and exposed. (B and C) Both [γ32P]8-N3ATP and [α32P]8-N3ATP predominantly photolabel split CFTR (arrow) at NBD1. (B) Autoradiogram from (not prephosphorylated) membranes of oocytes coexpressing Flag-3–835 and Flag-837–1480 (+) or from noninjected oocytes (−), incubated for 15 min at 30°C with 1 mM Vi and either 5 μM [α32P]8-N3ATP (left two lanes) or 5 μM [γ32P]8-N3ATP (right three lanes), followed by washes and 5-min postincubation at 30°C and then exposure to UV light (+) or darkness (−), before immunoprecipitation and SDS-PAGE. (C) Immunoprecipitates from the same membranes were immunoblotted with anti–R-domain antibody (left) or anti–COOH-terminal antibody (right).
Figure 7.
Little influence of phosphorylation with PKA on [α32P]8-N3ATP binding at 0°C (A) or tight binding/occlusion at 30°C (B and C). (A) Before the binding assay, HEK293T membranes expressing Flag-CFTR were incubated with (+PKA) or without (−PKA) PKA, washed twice with ice-cold nucleotide-free (and PKA-free) buffer, and then incubated for 5 min on ice with 0.5, 1, or 5 μM [α32P]8-N3ATP before UV irradiation without washes, immunoprecipitation, and SDS-PAGE to yield the autoradiogram shown. (B) Membranes were prephosphorylated with PKA (+) or not (−) and washed as in A, then incubated at 30°C for 15 min with 5 μM PKI and 5 μM [α32P]8-N3ATP ± 1 mM Vi (as indicated), washed as usual including a 5-min postincubation at 30°C, and then UV irradiated (except lane marked “−”) before immunoprecipitation, SDS-PAGE, and autoradiography. The right lane is a control using the same membranes but prephosphorylated with PKA and [γ32P]ATP, then washed and incubated with Vi for 15 min at 30°C like the other samples, but without [α32P]8-N3ATP, before washing, immunoprecipitation, SDS-PAGE, and autoradiography: the PKA-mediated phosphorylation of CFTR was retained throughout the incubation period with 8-N3 nucleotide. (C) PKA was included (+) or not (−) in the 10-min incubation of HEK293T-cell membranes expressing Flag-CFTR with 5 μM [α32P]8-N3ATP ± 1 mM Vi (as indicated) at 25°C, after which the membranes were washed as usual including a 5-min postincubation at 25°C, and then UV irradiated before immunoprecipitation, SDS-PAGE, and autoradiography.
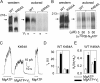
Figure 8.
Mutation K464A in CFTR impairs occlusion at 30°C, but not binding at 0°C, at low [nucleotide], and disrupts Vi-induced stabilization of open burst state. (A, left) Membranes of oocytes expressing WT or K464A CFTR (without Flag tags) were run on SDS-PAGE gels, transferred to nitrocellulose membranes, and blotted with anti–R-domain antibody. WT CFTR was expressed at least twice as well as K464A CFTR in this batch of oocytes (arrow marks mature fully-glycosylated CFTR; lower, sharper band is core-glycosylated CFTR). (Right) Autoradiogram showing nucleotide occlusion in the same membranes as at left, after incubation at 30°C for 15 min with 5 μM [α32P]8-N3ATP ± 1 mM Vi, washing including 5-min postincubation at 30°C, irradiation with UV, solubilization, and SDS-PAGE. Twice as much membrane was used for K464A samples as for WT samples. (B, left) Immunoblots of membranes from Flag-WT– and Flag-K464A–expressing oocytes (the Flag tags facilitated immunoprecipitation to enhanced signal-to-noise ratio, as membranes were not washed before photocrosslinking) blotted with anti–R-domain antibody as in A. The membranes contained about one third more WT than mutant K464A protein. (Right) Autoradiogram showing photolabeling of CFTR in the same membranes as at left after incubation with 5 or 50 μM [α32P]8-N3ATP for 5 min on ice, followed by UV irradiation, immunoprecipitation, SDS-PAGE, and autoradiography. Approximately 30% more membrane was used for Flag-K464A samples as for Flag-WT samples. (C) Macroscopic current in an oocyte patch containing hundreds of K464A CFTR channels. Current decays with comparable biexponential time course (superimposed fit lines largely obscured by data) after activation by 5 mM MgATP plus 300 nM PKA with 5 mM Vi (for trace shown: af = 23 pA, as = 7 pA, τf = 0.7 s, τs = 5.5 s) or without it (first decay af = 29 pA; as = 31 pA, τf = 0.5 s, τs = 1.4 s, second decay af = 57 pA; as = 5 pA, τf = 0.6 s, τs = 2.1 s). Solution exchange time <1 s. Bars beneath trace offset to signal different solution compositions. (D and E) Mean fit parameters for the time constant (D) and fractional amplitude (E) of the slow component of current decay after activation by 5 mM MgATP plus 300 nM PKA without (white bars) or with 5 mM Vi (black bars) for WT (n = 19) or K464A (n = 10).
Similar articles
- On the mechanism of MgATP-dependent gating of CFTR Cl- channels.
Vergani P, Nairn AC, Gadsby DC. Vergani P, et al. J Gen Physiol. 2003 Jan;121(1):17-36. doi: 10.1085/jgp.20028673. J Gen Physiol. 2003. PMID: 12508051 Free PMC article. - The two ATP binding sites of cystic fibrosis transmembrane conductance regulator (CFTR) play distinct roles in gating kinetics and energetics.
Zhou Z, Wang X, Liu HY, Zou X, Li M, Hwang TC. Zhou Z, et al. J Gen Physiol. 2006 Oct;128(4):413-22. doi: 10.1085/jgp.200609622. Epub 2006 Sep 11. J Gen Physiol. 2006. PMID: 16966475 Free PMC article. - Functional roles of nonconserved structural segments in CFTR's NH2-terminal nucleotide binding domain.
Csanády L, Chan KW, Nairn AC, Gadsby DC. Csanády L, et al. J Gen Physiol. 2005 Jan;125(1):43-55. doi: 10.1085/jgp.200409174. Epub 2004 Dec 13. J Gen Physiol. 2005. PMID: 15596536 Free PMC article. - ATP hydrolysis cycles and the gating of CFTR Cl- channels.
Gadsby DC, Dousmanis AG, Nairn AC. Gadsby DC, et al. Acta Physiol Scand Suppl. 1998 Aug;643:247-56. Acta Physiol Scand Suppl. 1998. PMID: 9789567 Review. - Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis.
Gadsby DC, Nairn AC. Gadsby DC, et al. Physiol Rev. 1999 Jan;79(1 Suppl):S77-S107. doi: 10.1152/physrev.1999.79.1.S77. Physiol Rev. 1999. PMID: 9922377 Review.
Cited by
- New structural insights into the gating movements of CFTR.
Puljung MC. Puljung MC. J Gen Physiol. 2015 May;145(5):365-9. doi: 10.1085/jgp.201511399. J Gen Physiol. 2015. PMID: 25918357 Free PMC article. No abstract available. - CFTR gating I: Characterization of the ATP-dependent gating of a phosphorylation-independent CFTR channel (DeltaR-CFTR).
Bompadre SG, Ai T, Cho JH, Wang X, Sohma Y, Li M, Hwang TC. Bompadre SG, et al. J Gen Physiol. 2005 Apr;125(4):361-75. doi: 10.1085/jgp.200409227. Epub 2005 Mar 14. J Gen Physiol. 2005. PMID: 15767295 Free PMC article. - Discovering the chloride pathway in the CFTR channel.
Farkas B, Tordai H, Padányi R, Tordai A, Gera J, Paragi G, Hegedűs T. Farkas B, et al. Cell Mol Life Sci. 2020 Feb;77(4):765-778. doi: 10.1007/s00018-019-03211-4. Epub 2019 Jul 20. Cell Mol Life Sci. 2020. PMID: 31327045 Free PMC article. - Review. ATP hydrolysis-driven gating in cystic fibrosis transmembrane conductance regulator.
Muallem D, Vergani P. Muallem D, et al. Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):247-55. doi: 10.1098/rstb.2008.0191. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18957373 Free PMC article. Review.
References
- Aleksandrov, A.A., and J.R. Riordan. 1998. Regulation of CFTR ion channel gating by MgATP. FEBS Lett. 431:97–101. - PubMed
- Aleksandrov, L., A. Mengos, X. Chang, A. Aleksandrov, and J.R. Riordan. 2001. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulador. J. Biol. Chem. 276:12918–12923. - PubMed
- Aleksandrov, L., A.A. Aleksandrov, X.B. Chang, and J.R. Riordan. 2002. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J. Biol. Chem. 277:15419–15425. - PubMed
- Baukrowitz, T., T.-C. Hwang, A.C. Nairn, and D.C. Gadsby. 1994. Coupling of CFTR Cl− channel gating to an ATP hydrolysis cycle. Neuron. 12:473–482. - PubMed
- Bear, C.E., C.H. Li, N. Kartner, R.J. Bridges, T.J. Jensen, M. Ramjeesingh, and J.R. Riordan. 1992. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell. 68:809–818. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials