Chemical communication among bacteria - PubMed (original) (raw)
. 2003 Nov 25;100 Suppl 2(Suppl 2):14549-54.
doi: 10.1073/pnas.1934514100. Epub 2003 Aug 29.
Affiliations
- PMID: 12949263
- PMCID: PMC304117
- DOI: 10.1073/pnas.1934514100
Chemical communication among bacteria
Michiko E Taga et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Cell-cell communication in bacteria is accomplished through the exchange of chemical signal molecules called autoinducers. This process, called quorum sensing, allows bacteria to monitor their environment for the presence of other bacteria and to respond to fluctuations in the number and/or species present by altering particular behaviors. Most quorum-sensing systems are species- or group-specific, which presumably prevents confusion in mixed-species environments. However, some quorum-sensing circuits control behaviors that involve interactions among bacterial species. These quorum-sensing circuits can involve both intra- and interspecies communication mechanisms. Finally, anti-quorumsensing strategies are present in both bacteria and eukaryotes, and these are apparently designed to combat bacteria that rely on cell-cell communication for the successful adaptation to particular niches.
Figures
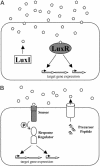
Fig. 1.
Canonical quorum-sensing circuits. (A) In typical Gram-negative LuxIR circuits, the LuxI-type protein catalyzes the synthesis of an AHL autoinducer (pentagons). The LuxR-type protein binds the AHL and controls the expression of target genes. (B) In typical Gram-positive AIP two-component quorumsensing circuits, precursor peptides are cleaved, modified, and exported by dedicated transporters. The resulting AIPs (circles) are detected by twocomponent sensor-histidine kinases, and sensory information is relayed by phosphorylation (P) of cognate response regulators that, in turn, control target gene expression. The proteins responsible for autoinducer binding are shaded in gray.
Fig. 2.
Structures of different autoinducers. (A Upper) AHL autoinducers share a common homoserine lactone moiety. (Lower) Side chains of some different AHLs are shown (R groups). The synthases and the organisms that produce them are listed. (B) AIP peptide sequences, their designations, and the organisms that produce them are shown. The asterisk above the tryptophan residue in ComX represents an isoprenyl group. (C) AI-2 of V. harveyi.
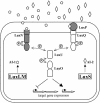
Fig. 3.
The V. harveyi quorum-sensing circuit. Two autoinducers, the AHL AI-1 (pentagons) and AI-2 (diamonds), are produced by their cognate synthases LuxLM and LuxS, respectively. AI-1 and AI-2 bind cognate sensors (LuxN and LuxPQ, respectively), initiating a phosphorylation cascade (P) that travels through LuxU and alters the phosphorylation state of the response regulator protein LuxO. LuxO controls the expression of target genes. The proteins responsible for autoinducer binding are shaded in gray.
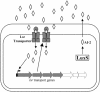
Fig. 4.
Lsr-mediated transport of AI-2 in S. typhimurium. AI-2 activates transcription of the lsr genes, four of which (shaded in gray) encode the Lsr transporter apparatus that functions to internalize AI-2.
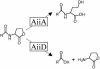
Fig. 5.
Enzymatic inactivation of AHLs. (Upper) AiiA of Bacillus species hydrolyzes the lactone ring of AHLs. (Lower) AiiD of a Ralstonia strain hydrolyzes AHLs to release the acyl side chain moiety and homoserine lactone.
References
- Miller, M. B. & Bassler, B. L. (2001) Annu. Rev. Microbiol. 55, 165-199. - PubMed
- Bassler, B. L. (1999) Curr. Opin. Microbiol. 2, 582-587. - PubMed
- Fuqua, C., Winans, S. C. & Greenberg, E. P. (1996) Annu. Rev. Microbiol. 50, 727-751. - PubMed
- Engebrecht, J., Nealson, K. & Silverman, M. (1983) Cell 32, 773-781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources