Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9 - PubMed (original) (raw)
. 2003 Sep 15;17(18):2298-307.
doi: 10.1101/gad.1111603. Epub 2003 Sep 2.
Affiliations
- PMID: 12952893
- PMCID: PMC196466
- DOI: 10.1101/gad.1111603
Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9
Paul M Ayton et al. Genes Dev. 2003.
Abstract
Transcriptional deregulation through the production of dominant-acting chimeric transcription factors derived from chromosomal translocations is a common theme in the pathogenesis of acute leukemias; however, the essential target genes for acute leukemogenesis are unknown. We demonstrate here that primary myeloid progenitors immortalized by various MLL oncoproteins exhibit a characteristic Hoxa gene cluster expression profile, which reflects that preferentially expressed in the myeloid clonogenic progenitor fraction of normal bone marrow. Continued maintenance of this MLL-dependent Hoxa gene expression profile is associated with conditional MLL-associated myeloid immortalization. Moreover, Hoxa7 and Hoxa9 were specifically required for efficient in vitro myeloid immortalization by an MLL fusion protein but not other leukemogenic fusion proteins. Finally, in a bone marrow transduction/transplantation model, Hoxa9 is essential for MLL-dependent leukemogenesis in vivo, a primary requirement detected at the earliest stages of disease initiation. Thus, a genetic reliance on Hoxa7 and Hoxa9 in MLL-mediated transformation demonstrates a gain-of-function mechanism for MLL oncoproteins as upstream constitutive activators that promote myeloid transformation via a Hox-dependent mechanism.
Figures
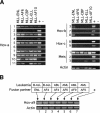
Figure 1.
Consistent expression of select Hox genes in murine and human cells transformed by MLL fusion genes. (A) RT-PCR analysis was conducted on RNA isolated from MPMP cell lines immortalized by the MLL fusion genes indicated at the top of the lanes. Primers employed for RT-PCR were specific for various Hox or Meis transcripts indicated to the left of the panels. (B) RT-PCR was performed on human cell lines that express MLL fusions with various partner proteins, which are indicated along with the leukemia subtypes at the top of the gel lanes. Cell lines are as follows: HB1119 (lane 1), RS411 (lane 2), MV4-11 (lane 3), ML21 (lane 4), MonoMac6 (lane 5), and THP1 (lane 6).
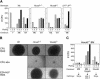
Figure 2.
Myeloid transformation requires sustained function of MLL-ENL and correlates with maintenance of Hox gene expression. (A) Myeloid clonogenic activity in normal bone marrow is associated with expression of Hoxa cluster genes. CFUs (per 10,000 plated cells) were determined after 7 d of culture in methylcellulose for whole bone marrow mononuclear cells (BM) or cellular fractions enriched based on the expression (Lin+) or absence (Lin-) of lineage markers. RT-PCR results are shown below for the Hox transcripts indicated to the right of each panel. (B) Conditional MLL-ENL proteins are schematically illustrated and consist of ER fusions at the C terminus of transformation-competent MLL-ENL (MER) or a mutant lacking the critical transformation domain of ENL (MEC). (C) Conditional immortalization of myeloid progenitors by MLL-ENL. Results of serial myeloid replating assays are shown for myeloid progenitors transduced with the constructs indicated at the top of the panels, cultured in the presence (+) or absence (-) of 4-OHT. Results of RT-PCR assays performed on cells harvested at day 7 of each plating are shown below for the transcripts indicated on the right. (D) MLL fusion protein activity is continuously required for the maintenance of transformation. A myeloid precursor cell line (MER3) immortalized by MLL/ENL-ER was plated in methylcellulose culture in the presence (+) or absence (-) of 4-OHT, and CFUs (per 10,000 plated cells) were determined on day 7 (lanes 1,2, respectively). Serial replating was performed as illustrated below, and CFUs again determined on day 7 (lanes 3,4, respectively).
Figure 3.
Hoxa7 and Hoxa9 are independently required for efficient in vitro immortalization by MLL-ENL. (A) Representative results (CFUs/10,000 plated cells) of serial myeloid replating assays are shown for myeloid progenitors transduced with vector alone (V), MLL-ENL (ME), or E2A-HLF (EH) as indicated. Genotypes of BM cells used for transductions are indicated above the panels. (B) Morphologies for colony types observed in methylcellulose assays initiated with cells transduced by MLL-ENL (rows 1 and 2) or E2A-HLF (row 3). Genotypes of transduced cells are indicated above the panels. (C) Coexpression of Hoxa9 in combination with MLL-ENL rescues in vitro immortalization of _Hoxa9_-/- BM cells. Results are shown as CFU/10,000 plated cells. Transduced constructs are indicated below, along with indications of ability to establish IL-3-dependent cell lines in liquid media.
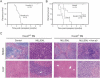
Figure 4.
Hoxa9 is essential for the leukemogenic properties of MLL-ENL. (A) Survival curves for cohorts of animals that were transplanted with MLL-ENL transduced progenitors from Hoxa9+/+ (n = 15) or _Hoxa9_-/- (n = 13) BM donors. (B) Coexpression of Hoxa9 in combination with MLL-ENL rescues in vivo leukemogenicity of _Hoxa9_-/- BM cells. Survival curves are shown for cohorts of animals that were transplanted with _Hoxa9_-/- progenitors transduced with Hoxa9 alone (n = 10), Hoxa9 + MLL-ENL (n = 10), or cell lines (lines 3 and 4) immortalized by coexpressed Hoxa9 and MLL-ENL. (C) Histology for spleens and livers from mice transplanted with transduced BM cells. Transduced genes and BM genotypes are indicated at the top.
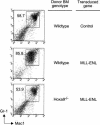
Figure 5.
_Hoxa9_-dependent preleukemia in mice transplanted with MLL-ENL transduced BM. BM cells were analyzed for expression of Gr-1 and Mac-1 at 5 wk posttransplant. Numbers of Gr-1+/Mac-1+ cells were increased in recipients of wild-type BM transduced with MLL-ENL but not _Hoxa9_-/- BM, which showed levels of Gr-1+/Mac-1+ cells similar to those of control mice. Donor BM genotypes and transduced genes are indicated to the right. FACS data are representative of two animals in each cohort.
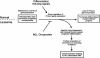
Figure 6.
Schematic model depicting Hoxa_-dependent myeloid transformation by MLL oncoproteins. Differentiation-inducing signals from the bone marrow microenvironment promote commitment of normal pluripotential HSCs toward terminal myeloid development that is associated with the loss of 5′_Hoxa gene expression. MLL oncoproteins disrupt this critical regulatory circuit, enhancing myeloid progenitor self-renewal via maintenance of 5′Hoxa gene expression, blocking differentiation events ultimately progressing to AML.
Similar articles
- Leukemic transformation of hematopoietic progenitors by MLL-GAS7 in the absence of Hoxa7 or Hoxa9.
So CW, Karsunky H, Wong P, Weissman IL, Cleary ML. So CW, et al. Blood. 2004 Apr 15;103(8):3192-9. doi: 10.1182/blood-2003-10-3722. Epub 2003 Dec 30. Blood. 2004. PMID: 15070702 - A carboxy-terminal domain of ELL is required and sufficient for immortalization of myeloid progenitors by MLL-ELL.
DiMartino JF, Miller T, Ayton PM, Landewe T, Hess JL, Cleary ML, Shilatifard A. DiMartino JF, et al. Blood. 2000 Dec 1;96(12):3887-93. Blood. 2000. PMID: 11090074 - The AF10 leucine zipper is required for leukemic transformation of myeloid progenitors by MLL-AF10.
DiMartino JF, Ayton PM, Chen EH, Naftzger CC, Young BD, Cleary ML. DiMartino JF, et al. Blood. 2002 May 15;99(10):3780-5. doi: 10.1182/blood.v99.10.3780. Blood. 2002. PMID: 11986236 - Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins.
Ayton PM, Cleary ML. Ayton PM, et al. Oncogene. 2001 Sep 10;20(40):5695-707. doi: 10.1038/sj.onc.1204639. Oncogene. 2001. PMID: 11607819 Review. - Deregulation of the HOXA9/MEIS1 axis in acute leukemia.
Collins CT, Hess JL. Collins CT, et al. Curr Opin Hematol. 2016 Jul;23(4):354-61. doi: 10.1097/MOH.0000000000000245. Curr Opin Hematol. 2016. PMID: 27258906 Free PMC article. Review.
Cited by
- miR-196b directly targets both HOXA9/MEIS1 oncogenes and FAS tumour suppressor in MLL-rearranged leukaemia.
Li Z, Huang H, Chen P, He M, Li Y, Arnovitz S, Jiang X, He C, Hyjek E, Zhang J, Zhang Z, Elkahloun A, Cao D, Shen C, Wunderlich M, Wang Y, Neilly MB, Jin J, Wei M, Lu J, Valk PJM, Delwel R, Lowenberg B, Le Beau MM, Vardiman J, Mulloy JC, Zeleznik-Le NJ, Liu PP, Zhang J, Chen J. Li Z, et al. Nat Commun. 2012 Feb 21;3:688. doi: 10.1038/ncomms1681. Nat Commun. 2012. PMID: 22353710 Free PMC article. - The impact of fusion genes on cancer stem cells and drug resistance.
Panicker S, Venkatabalasubramanian S, Pathak S, Ramalingam S. Panicker S, et al. Mol Cell Biochem. 2021 Oct;476(10):3771-3783. doi: 10.1007/s11010-021-04203-4. Epub 2021 Jun 7. Mol Cell Biochem. 2021. PMID: 34095988 Review. - Menin in Cancer.
Majer AD, Hua X, Katona BW. Majer AD, et al. Genes (Basel). 2024 Sep 21;15(9):1231. doi: 10.3390/genes15091231. Genes (Basel). 2024. PMID: 39336822 Free PMC article. Review. - Dimerization of MLL fusion proteins and FLT3 activation synergize to induce multiple-lineage leukemogenesis.
Ono R, Nakajima H, Ozaki K, Kumagai H, Kawashima T, Taki T, Kitamura T, Hayashi Y, Nosaka T. Ono R, et al. J Clin Invest. 2005 Apr;115(4):919-29. doi: 10.1172/JCI22725. Epub 2005 Mar 10. J Clin Invest. 2005. PMID: 15761502 Free PMC article. - Pathways, Processes, and Candidate Drugs Associated with a Hoxa Cluster-Dependency Model of Leukemia.
Kettyle LM, Lebert-Ghali CÉ, Grishagin IV, Dickson GJ, O'Reilly PG, Simpson DA, Bijl JJ, Mills KI, Sauvageau G, Thompson A. Kettyle LM, et al. Cancers (Basel). 2019 Dec 17;11(12):2036. doi: 10.3390/cancers11122036. Cancers (Basel). 2019. PMID: 31861091 Free PMC article.
References
- Armstrong S.A., Staunton, J.E., Silverman, L.B., Pieters, R., den Boer, M.L., Minden, M.D., Sallan, S.E., Lander, E.S., Golub, T.R., and Korsmeyer, S.J. 2002. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat. Genet. 30: 41-47. - PubMed
- Ayton P.M. and Cleary, M.L. 2001. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene 20: 5695-5707. - PubMed
- Ayton P., Sneddon, S.F., Palmer, D.B., Rosewell, I.R., Owen, M.J., Young, B., Presley, R., and Subramanian, V. 2001. Truncation of the Mll gene in exon 5 by gene targeting leads to early preimplantation lethality of homozygous embryos. Genesis 30: 201-212. - PubMed
- Buske C. and Humphries, R.K. 2000. Homeobox genes in leukemogenesis. Int. J. Hematol. 71: 301-308. - PubMed
- Chen F. and Capecchi, M.R. 1997. Targeted mutations in hoxa-9 and hoxb-9 reveal synergistic interactions. Dev. Biol. 181: 186-196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources