FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting - PubMed (original) (raw)
. 2003 Sep;112(5):683-92.
doi: 10.1172/JCI18399.
Michael T Collins, Neal S Fedarko, Natasha Cherman, Alessandro Corsi, Kenneth E White, Steven Waguespack, Anurag Gupta, Tamara Hannon, Michael J Econs, Paolo Bianco, Pamela Gehron Robey
Affiliations
- PMID: 12952917
- PMCID: PMC182207
- DOI: 10.1172/JCI18399
FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting
Mara Riminucci et al. J Clin Invest. 2003 Sep.
Abstract
FGF-23, a novel member of the FGF family, is the product of the gene mutated in autosomal dominant hypophosphatemic rickets (ADHR). FGF-23 has been proposed as a circulating factor causing renal phosphate wasting not only in ADHR (as a result of inadequate degradation), but also in tumor-induced osteomalacia (as a result of excess synthesis by tumor cells). Renal phosphate wasting occurs in approximately 50% of patients with McCune-Albright syndrome (MAS) and fibrous dysplasia of bone (FD), which result from postzygotic mutations of the GNAS1 gene. We found that FGF-23 is produced by normal and FD osteoprogenitors and bone-forming cells in vivo and in vitro. In situ hybridization analysis of FGF-23 mRNA expression identified "fibrous" cells, osteogenic cells, and cells associated with microvascular walls as specific cellular sources of FGF-23 in FD. Serum levels of FGF-23 were increased in FD/MAS patients compared with normal age-matched controls and significantly higher in FD/MAS patients with renal phosphate wasting compared with those without, and correlated with disease burden bone turnover markers commonly used to assess disease activity. Production of FGF-23 by FD tissue may play an important role in the renal phosphate-wasting syndrome associated with FD/MAS.
Figures
Figure 1
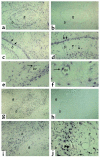
In situ hybridization analysis of FGF-23 expression in FD of bone. Biopsy specimens of FD-involved iliac crest. Serial sections from two FD phosphaturic patients were hybridized with antisense and sense RNA probes for FGF-23 mRNA. (a–f) Patient 1. (a and b) Expression of FGF-23 is clearly observed both in the fibrous component of the lesion and in bone cells associated with abnormal bone trabeculae (a). No signal is observed in sections hybridized with the sense (control) probe (b). (c and d) Strong expression of FGF-23 mRNA is observed in the abnormal bone cells associated with the surface of abnormal bone trabeculae (double arrows) and with individual osteocytes therein (arrowheads) (d). (e and f) Expression of FGF-23 mRNA is also observed in cells (endothelial cells and/or pericytes) within the walls of microvessels in the FD tissue (single arrows). (g–i) Patient 2. Strong hybridization signal in abnormal bone cells (g, i, j; thick arrows in j) and in spindle-shaped fibroblastic cells in the fibrous tissue (thin arrows in j). No signal is detected in sections hybridized with the sense probe (h). ft, fibrous tissue; b, bone; bv, blood vessel lumen.
Figure 2
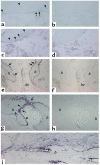
Immunohistochemical characterization of FGF-23–producing cells in FD. Undecalcified MMA sections (a and b) and paraffin sections (c–f) were prepared from the same tissue sample. (a and b) Von Kossa staining of MMA sections demonstrates the severe mineralization defect in FD bone, reflected in a significant excess of osteoid (ost) and paucity of mineralized bone matrix (mb; black with von Kossa). (c and d) Immunolocalization of ALP. Cells layered onto trabecular bone surfaces (double arrows in c and d) are intensely ALP positive. (e and f) FGF-23 in situ hybridization. The codistribution of the hybridization signal with the immunoreactivity for ALP (double arrows in c and e, respectively) is shown. Also note that osteocytes within FD bone do express FGF-23 (f), but are ALP negative (d).
Figure 3
Immunohistochemical characterization of FGF-23–producing cells in FD. Stromal cells in the fibrous tissue (ft) express FGF-23 mRNA (a), are intensely positive for the early markers of osteogenic differentiation, ALP (b) and ON (c), but are negative for the markers of late osteogenic differentiation, BSP (d) and OC (not shown). Both ALP and ON immunostaining result in decoration of a dense network of delicate cell processes of FD stromal cells. Mature osteogenic cells embedded as osteocytes in newly formed FD bone express the late marker of osteogenic differentiation, and OC (not shown) (e), and express FGF-23 mRNA (f).
Figure 4
Expression of FGF-23 in FD tissue from patients with normal phosphatemia and TmP/GFR. (a–c) Images are from one patient. (d–f) Images from a second patient. (c and f) Sections hybridized with the sense (control) probe. Signal for FGF-23 mRNA is observed in both cases (a and b, d and e).
Figure 5
In situ hybridization analysis of FGF-23 expression in normal bone. (a–d) Normal trabecular bone (iliac crest). (a and c) Antisense probe; (b and d) sense (control) probe. Hybridization signal is observed over flattened bone-lining cells (surface-associated quiescent osteogenic cells [a, arrows] and osteocytes [a and c, arrowheads]). (e–i) Normal fracture callus. bv, blood vessel lumen. (e, g, i) Antisense probe. (f and h) Sense (control) probe. Intense hybridization signal is observed in osteoblasts (e and g, arrows) actively involved in deposition of reparative bone (b) and in newly formed osteocytes therein (g, arrowheads). (i) Periosteum and subperiosteal bone. Note the expression of FGF-23 in periosteal osteoprogenitor cells (p).
Figure 6
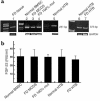
Expression of FGF-23 mRNA and protein. (a) RT-PCR primer sets that generate 251- and 373-bp products. FGF-23 mRNA was identified in fresh FD tissue (lane 1), in normal, nonclonal bone marrow stromal cells (BMSC) and multicolony-derived strains (MCDS) from a normal donor (lane 2), nonclonal strains of FD-derived stromal cells (comprising mutated and nonmutated cells) (lane 3), in pure strains of _GNAS1_-mutated stromal cells (FD 100% mut) (lane 4), and in HTB cells from normal (lanes 5 and 6) and FD (lane 7) donors. The identity of the amplification product was verified by DNA sequencing. (b) Conditioned medium from serum-free cultures of normal bone marrow stromal cells (BMSC), FD-derived nonclonal multicolony (mixed) strains and pure strains of _GNAS1_-mutated stromal cells (FD 100% mut), and normal and FD HTB cells (HTB) were harvested at 24 hours and analyzed by ELISA for production of FGF-23. Measurable levels of FGF-23 were observed in all cultures, with no significant difference between _GNAS1_-mutation status or type of cell culture. The values represent the combined results of two separate experiments and error bars represent standard error of the mean. Standard medium (containing 15% FBS) does not contain detectable FGF-23. RIU, relative immunoreactive unit.
Figure 7
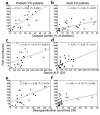
Negative correlation of serum FGF-23 levels with serum phosphate (a and b) and TmP/GFR (c and d) in pediatric and adult FD patients.
Figure 8
Positive correlation of serum FGF-23 with disease burden assessed as percentage of skeletal involvement in bone scintigrams (a and b) and with markers often used to assess FD activity, ALP (c and d) and deoxypyridinoline (e and f), for pediatric and adult FD patients. CR, creatinine.
Comment in
- Evidence for a bone-kidney axis regulating phosphate homeostasis.
Quarles LD. Quarles LD. J Clin Invest. 2003 Sep;112(5):642-6. doi: 10.1172/JCI19687. J Clin Invest. 2003. PMID: 12952909 Free PMC article.
Similar articles
- Expression of FGF23 is correlated with serum phosphate level in isolated fibrous dysplasia.
Kobayashi K, Imanishi Y, Koshiyama H, Miyauchi A, Wakasa K, Kawata T, Goto H, Ohashi H, Koyano HM, Mochizuki R, Miki T, Inaba M, Nishizawa Y. Kobayashi K, et al. Life Sci. 2006 Apr 11;78(20):2295-301. doi: 10.1016/j.lfs.2005.09.052. Epub 2005 Dec 7. Life Sci. 2006. PMID: 16337659 - Fibrous dysplasia, phosphate wasting and fibroblast growth factor 23.
Imel EA, Econs MJ. Imel EA, et al. Pediatr Endocrinol Rev. 2007 Aug;4 Suppl 4:434-9. Pediatr Endocrinol Rev. 2007. PMID: 17982392 Review. - Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia.
Collins MT, Chebli C, Jones J, Kushner H, Consugar M, Rinaldo P, Wientroub S, Bianco P, Robey PG. Collins MT, et al. J Bone Miner Res. 2001 May;16(5):806-13. doi: 10.1359/jbmr.2001.16.5.806. J Bone Miner Res. 2001. PMID: 11341325 - FGF23 and disorders of phosphate homeostasis.
Yu X, White KE. Yu X, et al. Cytokine Growth Factor Rev. 2005 Apr;16(2):221-32. doi: 10.1016/j.cytogfr.2005.01.002. Epub 2005 Feb 5. Cytokine Growth Factor Rev. 2005. PMID: 15863037 Review. - Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice.
Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Jüppner H, Lanske B. Sitara D, et al. Matrix Biol. 2004 Nov;23(7):421-32. doi: 10.1016/j.matbio.2004.09.007. Matrix Biol. 2004. PMID: 15579309 Free PMC article.
Cited by
- FGF-23, Left Ventricular Hypertrophy, and Mortality in Patients With CKD: A Revisit With Mediation Analysis.
Hidaka N, Inoue K, Kato H, Hoshino Y, Koga M, Kinoshita Y, Takashi Y, Makita N, Fukumoto S, Nangaku M, Ito N. Hidaka N, et al. JACC Adv. 2023 Dec 9;3(1):100747. doi: 10.1016/j.jacadv.2023.100747. eCollection 2024 Jan. JACC Adv. 2023. PMID: 38939808 Free PMC article. - Crosstalk between bone and brain in Alzheimer's disease: Mechanisms, applications, and perspectives.
Liu ZT, Liu MH, Xiong Y, Wang YJ, Bu XL. Liu ZT, et al. Alzheimers Dement. 2024 Aug;20(8):5720-5739. doi: 10.1002/alz.13864. Epub 2024 Jun 2. Alzheimers Dement. 2024. PMID: 38824621 Free PMC article. Review. - Non-Classical Effects of FGF23: Molecular and Clinical Features.
Martínez-Heredia L, Canelo-Moreno JM, García-Fontana B, Muñoz-Torres M. Martínez-Heredia L, et al. Int J Mol Sci. 2024 Apr 30;25(9):4875. doi: 10.3390/ijms25094875. Int J Mol Sci. 2024. PMID: 38732094 Free PMC article. Review. - Transcriptomic Signature and Pro-Osteoclastic Secreted Factors of Abnormal Bone-Marrow Stromal Cells in Fibrous Dysplasia.
Michel Z, Raborn LN, Spencer T, Pan KS, Martin D, Roszko KL, Wang Y, Robey PG, Collins MT, Boyce AM, de Castro LF. Michel Z, et al. Cells. 2024 Apr 30;13(9):774. doi: 10.3390/cells13090774. Cells. 2024. PMID: 38727310 Free PMC article.
References
- DiMeglio LA, White KE, Econs MJ. Disorders of phosphate metabolism. Endocrinol. Metab. Clin. North Am. 2000;29:591–609. - PubMed
- The ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF-23. The ADHR Consortium. Nat. Genet. 2000;26:345–348. - PubMed
- White K, et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. - PubMed
- Shimada T, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. - PubMed
- Weidner N. Review and update: oncogenic osteomalacia-rickets. Ultrastruct. Pathol. 1991;15:317–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR-47866/AR/NIAMS NIH HHS/United States
- R01 AR047866/AR/NIAMS NIH HHS/United States
- P01 AG018397/AG/NIA NIH HHS/United States
- AG-18397/AG/NIA NIH HHS/United States
- E.1029/TI_/Telethon/Italy
- R01 AR042228/AR/NIAMS NIH HHS/United States
- R01 DK063934/DK/NIDDK NIH HHS/United States
- AR-02095/AR/NIAMS NIH HHS/United States
- AR-42228/AR/NIAMS NIH HHS/United States
- DK-063934/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases