A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397 - PubMed (original) (raw)
A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397
Qi Shi et al. Mol Biol Cell. 2003 Oct.
Abstract
The common model for integrin mediated signaling is based on integrin clustering and the potential for that clustering to recruit signaling molecules including FAK and src. The clustering model for transmembrane signaling originated with the analysis of the EGF receptor signaling and remains the predominant model. The roles for substrate-bound ligand and ligand occupancy in integrin-mediated signaling are less clear. A kinetic model was established using HT1080 cells in which there was a linear relationship between the strength of adhesion, the proportion of alpha5beta1 integrin that could be chemically cross-linked, and the number of receptor-ligand bonds. This graded signal produced a similarly graded response measured by the level of specific phosphorylation of FAK Y397. FAK Y397 phosphorylation could also be induced by antibody bound to the substrate. In contrast, clustering of alpha5beta1 on suspended cells with either antibody to beta1 or by clustering of soluble ligand bound to alpha5beta1 induced the phosphorylation of FAK Y861 but not Y397. There were no differences in signaling when activating antibodies were compared with blocking antibodies, presence or absence of ligand. Only tethering of alpha5beta1 to the substrate was required for induction of FAK Y397 phosphorylation.
Figures
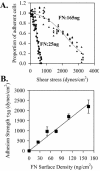
Figure 1.
Adhesion strength of HT1080 cells to fibronectin. HT1080 cells were incubated in serum-free medium for 18 h, washed, dissociated with EDTA, and plated on fibronectin-coated, BSA-blocked, surfaces in complete PBS plus 2 mM glucose for 1 h at room temperature. Cells were analyzed using the spinning disk system in adhesion buffer containing 5% Dextran: (A) Plated on fibronectin density 25 ng/cm2 (•) or 165 ng/cm2 (▴). (B) Adhesion strength defined as mean detachment shear stress (τ50) from spinning disk analyses as shown in A were plotted as a function of fibronectin surface density as determined by 125I adsorption (Garcia et al., 1998b). Error bars, SD; n = 3.
Figure 2.
Proportion of α5β1 bound to fibronectin as a function of density. HT1080 cells were incubated in serum-free medium for 18 h, washed, dissociated with EDTA, and plated on fibronectin-coated, BSA-blocked, surfaces in complete PBS plus 2 mM glucose for 1 h at room temperature. The cultures were then cross-linked with sulfo-BSCOES for 10 min and the cross-linked and non–cross-linked fractions were analyzed by Western blot. (A) Cross-linked α5; typical Western blot and combined quantification for three independent experiments. Bound integrin subunits represents the proportion of α5 cross-linked/total α5 × 100. Linear regression: _R_2 = 0.98, p = 0.001. (B) Cross-linked β1. Same as A. Error bars, SEM for n = 3 separate experiments. Linear regression: _R_2 = 0.99, p = 0.0005. (C) Adhesion strength τ50 taken from Figure 1B plotted against amount of cross-linked α5 from Figure 2A shows the linear relationship.
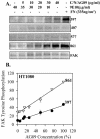
Figure 3.
Phosphorylation of FAK by α5β1 binding to substrate fibronectin in HT1080 cells. HT1080 cells were incubated in serum-free medium for 18 h, washed, dissociated with EDTA, and plated on fibronectin-coated, BSA-blocked, surfaces in complete PBS plus 2 mM glucose for 1 h at room temperature. The cells were extracted with a RIPA-vanadate buffer and analyzed by Western blot using antibodies to specific FAK phosphorylation sites or a mAb to FAK protein. (A) A representative blot; (B) the quantified combined data from three independent experiments FAK tyrosine phosphorylation (level of FAK detected by the specific antiphosphotyrosine antibody/level of FAK protein for each data point ×10). Error bars, SEM for n = 3 separate experiments. Linear regression: pY397 _R_2 = 0.98, p = 0.0009; pY407 _R_2 = 0.66, p = 0.09; pY577 _R_2 = 0.87, p = 0.02; pY861 _R_2 = 0.99, p = 0.0004. (C) The data as a function of the level of cross-linked α5. Linear regression: pY397 _R_2 = 0.99, p <0.0001; pY407 _R_2 = 0.72, p = 0.07; pY577 _R_2 = 0.84, p = 0.03; pY861 _R_2 = 0.97, p = 0.0017.
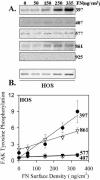
Figure 4.
Phosphorylation of FAK by α5β1 binding to substrate fibronectin in HOS cells. Similar to Figure 3 except experiments shown are for HOS cells. (A) A representative blot. (B) The quantified combined data from three independent experiments FAK tyrosine phosphorylation. Error bars, SEM for n = 3 separate experiments. Linear regression: pY397 _R_2 = 0.94, p = 0.0059; pY407 _R_2 = 0.74, p = 0.06; pY577 _R_2 = 0.81, p = 0.0382; pY861 _R_2 = 0.97, p = 0.0027.
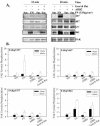
Figure 5.
FAK Y397 phosphorylation induction by α5β1 and αvβ3 is HT1080 cells and HOS cells is different. (A) Cross-linking of αv; linear regression: _R_2 = 0.95, p = 0.0046, and (B) β3 for HT1080 cells platted fibronectin using the protocol in Figure 2; linear regression: _R_2 = 0.96, p = 0.0032. (C) The inhibition of FAK Y397 phosphorylation by antibodies to α5β1 and/or αvβ3 at both 10 and 60 min after plating for HT1080 cells. (D) The inhibition of FAK Y397 phosphorylation by antibodies to α5β1 and/or αvβ3 at both 10 and 60 min after plating for HOS cells. Error bars, SEM for n = 3 for each.
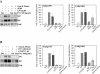
Figure 6.
Phosphorylation of FAK by clustering integrin. HT1080 cells were incubated in serum-free medium for 18 h, washed, and dissociated with EDTA. Some samples were mixed with the mAb AIIB2, or AIIB2 plus secondary goat anti-rat IgG and plated on fibronectin-coated (335 ng/cm2), BSA-blocked surfaces in complete PBS plus 2 mM glucose for 10 and 60 min at room temperature. (A) A typical Western blot; (B) the quantification of Western blots for specific phosphorylated FAK; (phospho-specific antibody/total FAK) for each. Values were normalized to the phosphorylation level of the suspended cells in the absence of cluster inducing antibodies. Error bars, SEM for n = 3.
Figure 7.
FAK stimulation by activated or ligand occupied α5β1. (A) Serum-starved HT1080 cells were treated is suspension with the β1 activating mAb AG89 or AG89 plus secondary anti-mouse IgG, or AG89 was bound to a nitrocellulose-coated surface. Cells kept in suspension or plated on fibronectin were used as controls. The specific phosphorylation is shown only for Y397 and Y861, there were no changes in Y407 or Y577. Data were normalized setting the specific phosphorylation for cells plated on fibronectin at 100%; error bars, SEM for n = 3. (B) Serum-starved HT1080 cells were treated in suspension with FN7–10 (40 μg/ml) and MnCl2 (1 mM) for 15 min; 13G12 (10 mg/ml) was added followed by secondary goat anti-mouse IgG (10 μg/ml) for 60 min. For controls, cells were maintained in suspension, plated on fibronectin, or plated on FN7–10 bound to 13G12 bound to nitrocellulose-coated surfaces. Quantification as for A, Error bars, SEM for n = 3.
Figure 8.
Dose-dependent tethering stimulation of FAK phosphorylation. Serum-starved HT1080 cells were plated on antibody substrates formed from the binding of mixtures of AG89 diluted with different proportions of 9E10 that were bound to nitrocellulose-coated surfaces. Specific phosphorylation of Y861 and Y397 is shown as a function of antibody-ligand density. Error bars, SEM for n = 3. Linear regression: pY397 _R_2 = 0.96, p < 0.0001; pY861 _R_2 = 0.99, p < 0.0001.
Similar articles
- Transformation of chicken embryo fibroblasts by v-src uncouples beta1 integrin-mediated outside-in but not inside-out signaling.
Datta A, Shi Q, Boettiger DE. Datta A, et al. Mol Cell Biol. 2001 Nov;21(21):7295-306. doi: 10.1128/MCB.21.21.7295-7306.2001. Mol Cell Biol. 2001. PMID: 11585912 Free PMC article. - CCN2 promotes keratinocyte adhesion and migration via integrin α5β1.
Kiwanuka E, Andersson L, Caterson EJ, Junker JP, Gerdin B, Eriksson E. Kiwanuka E, et al. Exp Cell Res. 2013 Nov 15;319(19):2938-46. doi: 10.1016/j.yexcr.2013.08.021. Epub 2013 Aug 26. Exp Cell Res. 2013. PMID: 23988606 - Sulfatide interacts with and activates integrin αVβ3 in human hepatocellular carcinoma cells.
Wang R, Qi B, Dong YW, Cai QQ, Deng NH, Chen Q, Li C, Jin YT, Wu XZ. Wang R, et al. Oncotarget. 2016 Jun 14;7(24):36563-36576. doi: 10.18632/oncotarget.9095. Oncotarget. 2016. PMID: 27145276 Free PMC article. - Integrins in cell adhesion and signaling.
Akiyama SK. Akiyama SK. Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
Cited by
- β1-Integrin cytoskeletal signaling regulates sensory neuron response to matrix dimensionality.
Ribeiro A, Balasubramanian S, Hughes D, Vargo S, Powell EM, Leach JB. Ribeiro A, et al. Neuroscience. 2013 Sep 17;248:67-78. doi: 10.1016/j.neuroscience.2013.05.057. Epub 2013 Jun 10. Neuroscience. 2013. PMID: 23764511 Free PMC article. - Adrenomedullin expression in epithelial ovarian cancers and promotes HO8910 cell migration associated with upregulating integrin α5β1 and phosphorylating FAK and paxillin.
Deng B, Zhang S, Miao Y, Han Z, Zhang X, Wen F, Zhang Y. Deng B, et al. J Exp Clin Cancer Res. 2012 Mar 9;31(1):19. doi: 10.1186/1756-9966-31-19. J Exp Clin Cancer Res. 2012. PMID: 22400488 Free PMC article. - Suppression of uPAR retards radiation-induced invasion and migration mediated by integrin β1/FAK signaling in medulloblastoma.
Nalla AK, Asuthkar S, Bhoopathi P, Gujrati M, Dinh DH, Rao JS. Nalla AK, et al. PLoS One. 2010 Sep 24;5(9):e13006. doi: 10.1371/journal.pone.0013006. PLoS One. 2010. PMID: 20886051 Free PMC article. - Spatiotemporal constraints on the force-dependent growth of focal adhesions.
Stricker J, Aratyn-Schaus Y, Oakes PW, Gardel ML. Stricker J, et al. Biophys J. 2011 Jun 22;100(12):2883-93. doi: 10.1016/j.bpj.2011.05.023. Biophys J. 2011. PMID: 21689521 Free PMC article. - Focal adhesion kinase: from biological functions to therapeutic strategies.
Tan X, Yan Y, Song B, Zhu S, Mei Q, Wu K. Tan X, et al. Exp Hematol Oncol. 2023 Sep 25;12(1):83. doi: 10.1186/s40164-023-00446-7. Exp Hematol Oncol. 2023. PMID: 37749625 Free PMC article. Review.
References
- Akiyama, S.K., Aota, S., and Yamada, K.M. (1995). Function and receptor specificity of a minimal 20 kilodalton cell adhesive fragment of fibronectin. Cell Adhes. Commun. 3, 13–25. - PubMed
- Arthur, W.T., Petch, L.A., and Burridge, K. (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719–722. - PubMed
- Ballesteros, J.A., Jensen, A.D., Liapakis, G., Rasmussen, S.G., Shi, L., Gether, U., Javitch, J.A. (2001). Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 276, 29171–29177. - PubMed
- Boettiger, D., George-Weinstein, M., Menko, A. S. (1989). Triggering terminal myogenic differentiation. In: UCLA Symposia on Mol. Cell. Biol., New Series: Cellular and Molecular Biology of Muscle Development, ed. F. Stockdale, L. Kedes, New York: Alan R. Liss, Inc., 57–66.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous