Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1 - PubMed (original) (raw)
Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1
Victoria C Foletta et al. J Cell Biol. 2003.
Erratum in
- J Cell Biol. 2003 Oct 27;163(2):421. Soosairaiah Juliana [corrected to Soosairajah Juliana]
Abstract
Bone morphogenetic proteins (BMPs) regulate multiple cellular processes, including cell differentiation and migration. Their signals are transduced by the kinase receptors BMPR-I and BMPR-II, leading to Smad transcription factor activation via BMPR-I. LIM kinase (LIMK) 1 is a key regulator of actin dynamics as it phosphorylates and inactivates cofilin, an actin depolymerizing factor. During a search for LIMK1-interacting proteins, we isolated clones encompassing the tail region of BMPR-II. Although the BMPR-II tail is not involved in BMP signaling via Smad proteins, mutations truncating this domain are present in patients with primary pulmonary hypertension (PPH). Further analysis revealed that the interaction between LIMK1 and BMPR-II inhibited LIMK1's ability to phosphorylate cofilin, which could then be alleviated by addition of BMP4. A BMPR-II mutant containing the smallest COOH-terminal truncation described in PPH failed to bind or inhibit LIMK1. This study identifies the first function of the BMPR-II tail domain and suggests that the deregulation of actin dynamics may contribute to the etiology of PPH.
Figures
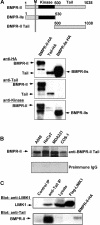
Figure 1.
Immunoprecipitation analyses of overexpressed LIMK1 and its interaction with BMPR-II proteins in COS-7 cells. (A) Schematic representation of full-length and truncated BMPR-II proteins. The extracellular domain (gray), the transmembrane and kinase domains (small and large black areas, respectively), and the cytoplasmic tail (white) are presented. The numbers of the amino acid residues are indicated above each structure as well as the site of the most COOH-terminal mutation currently identified in PPH patients, R873X. (B) Immunoprecipitation and immunoblot analyses of GFP-tagged LAPs interacting with FLAG-tagged LIMK1 (F-LIMK1) but not FLAG–Btk (F-Btk). (C) GST-tagged LIMK1 interaction with full-length myc-tagged BMPR-II (M-BMPR-II) or FLAG-tagged truncated BMPR-II (F-BMPR-II-T; contains no cytoplasmic tail).
Figure 2.
Interaction between endogenous LIMK1 and BMPR-II. (A) Schematic representation of BMPR-II, the short alternatively spliced isoform BMPR-IIs and the COOH-terminal tail construct, and recognition of these proteins, expressed in COS-7 cells as HA-tagged constructs, by anti–kinase domain and anti-tail domain antibodies. M, transmembrane region. (B) Recognition of endogenous BMPR-II by anti–BMPR-II-tail antibodies in Western immunoblotting of lysates from the indicated cell lines. (C) Association of endogenous BMPR-II and LIMK1 in NIH3T3 cells. Lysates from NIH3T3 cells were immunoprecipitated with anti–BMPR-II-tail antibodies, and the presence of BMPR-II and LIMK1 in the immunocomplexes was determined by Western immunoblotting with the indicated antibodies. Two percent of the total lysate used for immunoprecipitation was tested by immunoblotting for LIMK1 expression (Lysate). FLAG-tagged LIMK1 and HA-tagged BMPR-II immunoprecipitated from transfected COS-7 cells with antibodies against these epitopes served as marker controls.
Figure 3.
Analysis of LIMK1 and BMPR-II interaction. (A) GST–LIMK1 interaction with myc-tagged LAP proteins (M-hLAP15s and M-mLAP16s); isolated regions of the cytoplasmic tail of BMPR-II. (B) GST–LIMK1 interaction with wild-type, untagged BMPR-II and mutated BMPR-II (B-R873X) containing a COOH-terminal mutation (see Fig. 1 A). (C) FLAG-tagged LIM and PDZ domains of LIMK1 (F-LIM1,2 and F-PDZ) and full-length F-LIMK1, but not FLAG-tagged LIMK1 kinase domain (F-KIN) or F-Btk, interact with full-length BMPR-II. A schematic diagram of the LIMK1 domains is represented below the panels. The LIM domains (dark gray), PDZ domain (black), and kinase region (light gray) are indicated. (D) Association of two different amounts (10 and 20 μl) of GST–LIMK1 bound to glutathione-Sepharose beads with HA–PAK4 (lanes 1 and 4), BMPR-II (lanes 2 and 5), and both PAK4 and BMPR-II (lanes 3 and 6). Numbers above the blots indicate the fold change in HA–PAK4's ability to bind GST–LIMK1 in the presence and absence of overexpressed BMPR-II.
Figure 4.
Inhibition of LIMK1 function after interaction with BMPR-II. (A) In vitro kinase assay of coimmunoprecipitated myc-tagged LIMK1 and BMPR-II (M-LIMK1 and M-BMPR-II) proteins using 5 μg of GST–cofilin as substrate. Autophosphorylated M-LIMK1 and M-BMPR-II and phosphorylated GST–cofilin (arrowheads) and their level of expression as determined by immunoblotting (arrows) are indicated at the top and bottom panels, respectively. The level of cofilin phosphorylation is an indication of LIMK1 activity, and the fold change in cofilin phosphorylation was calculated by PhosphorImage analysis after normalization for the level of LIMK1 expression as determined by immunoblotting. The level of cofilin phosphorylation in the presence of LIMK1 alone was used as the baseline and designated 1.0. (B) In vitro kinase assays of coimmunoprecipitated FLAG- or GFP-tagged LIMK1 (F- or GFP-LIMK1) or GFP-tagged kinase domain of LIMK1 (GFP-KIN) with or without M-BMPR-II. (C) In vitro kinase assays of immunoprecipitated GFP–LIMK1 in the presence or absence of tailless kinase dead BMPR-II (BMPRII-T-KD). 5 μg of GST–cofilin is used as substrate in all samples. Phosphorylated proteins (arrowheads, top panel) and their level of expression after immunoblotting (arrows, bottom panel) are indicated. The fold change in GST–cofilin phosphorylation by LIMK1 was calculated as described above.
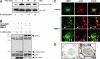
Figure 5.
Effects of BMP4 on LIMK1 activity and subcellular localization. (A) Immunoblot of COS cell lysates before and after stimulation with 10 ng/ml BMP4. The membrane was probed with anti–phospho-cofilin, stripped, and reprobed with anti-cofilin antibodies. The numbers below indicate the fold induction of phospho-cofilin level after BMP4 stimulation and were adjusted for the level of cofilin in the lysates. (B) Immunoblots of cell lysates prepared from COS cells overexpressing GST–LIMK1, BMPR-II, and both GST–LIMK1 and BMPR-II after or before BMP4 stimulation. The filters were probed with anti–BMPR-II (tail), anti-LIMK1 (rat monoclonal), anti–phospho-cofilin, and anti-cofilin antibodies. The numbers below indicate the fold induction of phospho-cofilin (P-cofilin) level after BMP4 stimulation and were adjusted for the level of cofilin in the lysates. The levels of overexpressed GST–LIMK1 and BMPR-II were consistently much higher when expressed separately than when coexpressed in the same cells. (C) Immunofluorescence analysis of endogenous LIMK1 and actin colocalization in unstimulated COS-7 cells (top) and in COS-7 cells stimulated with 100 ng/ml BMP4 for 10 min (middle and bottom). Arrowheads highlight the coredistribution of LIMK1 and F-actin to the cell's peripheral ruffles. Bar, 20 μM. (D) Immunohistochemical analysis of endogenous LIMK1 expression in the precapillary pulmonary artery of normal human lung (i) and in lung tissue from an individual with PPH (ii).
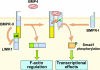
Figure 6.
Schematic representation of two signaling outputs from the BMPR system. In the canonical Smad signaling process, BMP4 binds to and brings together BMPR-I (-IA or -IB, also known as ALK3 and ALK6, respectively) and BMPR-II. BMPR-II phosphorylates the regulatory region (green box) of BMPR-I, activating the kinase domain (blue box), which phosphorylates Smad1, leading to its nuclear translocation for regulation of target genes. The present results show that the COOH-terminal tail domain (yellow box) of BMPR-II in the basal state binds to the LIM domain region of LIMK1 and inhibits LIMK1. BMP4 binding relieves this inhibitory interaction, enabling LIMK1 to phosphorylate cofilin, thereby regulating the actin cytoskeleton.
Similar articles
- Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis.
Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, Attisano L. Lee-Hoeflich ST, et al. EMBO J. 2004 Dec 8;23(24):4792-801. doi: 10.1038/sj.emboj.7600418. Epub 2004 Nov 11. EMBO J. 2004. PMID: 15538389 Free PMC article. - Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension.
Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Rudarakanchana N, et al. Hum Mol Genet. 2002 Jun 15;11(13):1517-25. doi: 10.1093/hmg/11.13.1517. Hum Mol Genet. 2002. PMID: 12045205 - Functional heterogeneity of bone morphogenetic protein receptor-II mutants found in patients with primary pulmonary hypertension.
Nishihara A, Watabe T, Imamura T, Miyazono K. Nishihara A, et al. Mol Biol Cell. 2002 Sep;13(9):3055-63. doi: 10.1091/mbc.e02-02-0063. Mol Biol Cell. 2002. PMID: 12221115 Free PMC article. - The crystal structure of the BMP-2:BMPR-IA complex and the generation of BMP-2 antagonists.
Nickel J, Dreyer MK, Kirsch T, Sebald W. Nickel J, et al. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S7-14. J Bone Joint Surg Am. 2001. PMID: 11263668 Review. - Bone morphogenetic proteins, genetics and the pathophysiology of primary pulmonary hypertension.
De Caestecker M, Meyrick B. De Caestecker M, et al. Respir Res. 2001;2(4):193-7. doi: 10.1186/rr57. Epub 2001 Jun 11. Respir Res. 2001. PMID: 11686884 Free PMC article. Review.
Cited by
- Functions of RNA-Binding Proteins in Cardiovascular Disease.
Ruffenach G, Medzikovic L, Sun W, Hong J, Eghbali M. Ruffenach G, et al. Cells. 2023 Dec 8;12(24):2794. doi: 10.3390/cells12242794. Cells. 2023. PMID: 38132114 Free PMC article. Review. - Idiopathic and heritable PAH perturb common molecular pathways, correlated with increased MSX1 expression.
Austin ED, Menon S, Hemnes AR, Robinson LR, Talati M, Fox KL, Cogan JD, Hamid R, Hedges LK, Robbins I, Lane K, Newman JH, Loyd JE, West J. Austin ED, et al. Pulm Circ. 2011 Jul-Sep;1(3):389-98. doi: 10.4103/2045-8932.87308. Pulm Circ. 2011. PMID: 22140629 Free PMC article. - High-altitude pulmonary hypertension in cattle (brisket disease): Candidate genes and gene expression profiling of peripheral blood mononuclear cells.
Newman JH, Holt TN, Hedges LK, Womack B, Memon SS, Willers ED, Wheeler L, Phillips JA 3rd, Hamid R. Newman JH, et al. Pulm Circ. 2011 Oct-Dec;1(4):462-9. doi: 10.4103/2045-8932.93545. Pulm Circ. 2011. PMID: 22530101 Free PMC article. - BMP signaling specifies the development of a large and fast CNS synapse.
Xiao L, Michalski N, Kronander E, Gjoni E, Genoud C, Knott G, Schneggenburger R. Xiao L, et al. Nat Neurosci. 2013 Jul;16(7):856-64. doi: 10.1038/nn.3414. Epub 2013 May 26. Nat Neurosci. 2013. PMID: 23708139 - Constitutive activation of BMP signalling abrogates experimental metastasis of OVCA429 cells via reduced cell adhesion.
Shepherd TG, Mujoomdar ML, Nachtigal MW. Shepherd TG, et al. J Ovarian Res. 2010 Feb 26;3:5. doi: 10.1186/1757-2215-3-5. J Ovarian Res. 2010. PMID: 20187934 Free PMC article.
References
- Aberle, H., A.P. Haghighi, R.D. Fetter, B.D. McCabe, T.R. Magalhaes, and C.S. Goodman. 2002. Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 33:545–558. - PubMed
- Allan, D.W., S.E. Pierre, I. Miguel-Aliaga, and S. Thor. 2003. Specification of neuropeptide cell identity by the integration of retrograde BMP signaling and a combinatorial transcription factor code. Cell. 113:73–86. - PubMed
- Arber, S., F.A. Barbayannis, H. Hanser, C. Schneider, C.A. Stanyon, O. Bernard, and P. Caroni. 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 393:805–809. - PubMed
- Bernard, O., S. Ganiatsas, G. Kannourakis, and R. Dringen. 1994. Kiz-1, a protein with LIM zinc finger and kinase domains, is expressed mainly in neurons. Cell Growth Differ. 5:1159–1171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous