Nup358 integrates nuclear envelope breakdown with kinetochore assembly - PubMed (original) (raw)
Nup358 integrates nuclear envelope breakdown with kinetochore assembly
Davide Salina et al. J Cell Biol. 2003.
Abstract
Nuclear envelope breakdown (NEBD) and release of condensed chromosomes into the cytoplasm are key events in the early stages of mitosis in metazoans. NEBD involves the disassembly of all major structural elements of the nuclear envelope, including nuclear pore complexes (NPCs), and the dispersal of nuclear membrane components. The breakdown process is facilitated by microtubules of the mitotic spindle. After NEBD, engagement of spindle microtubules with chromosome-associated kinetochores leads to chromatid segregation. Several NPC subunits relocate to kinetochores after NEBD. siRNA-mediated depletion of one of these proteins, Nup358, reveals that it is essential for kinetochore function. In the absence of Nup358, chromosome congression and segregation are severely perturbed. At the same time, the assembly of other kinetochore components is strongly inhibited, leading to aberrant kinetochore structure. The implication is that Nup358 plays an essential role in integrating NEBD with kinetochore maturation and function. Mitotic arrest associated with Nup358 depletion further suggests that mitotic checkpoint complexes may remain active at nonkinetochore sites.
Figures
Figure 1.
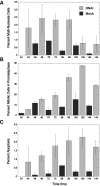
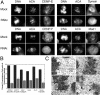
Mitotic defects associated with Nup358 siRNA treatment of HeLa cells. Nup358 siRNA treatment of HeLa cells leads to the appearance of cells containing multiple micronuclei over a period of 24–96 h (A) as well as the accumulation of cells arrested in prometaphase (B). The latter reach a peak at 120 h, at which time they account for almost 50% of the total mitotic population (B). A gradual increase in apoptosis, peaking at 120 h, is also observed (C). This increase in apoptosis may account for the decline in the number of multinucleate cells observed after 96 h of Nup358 siRNA treatment. In each graph, siRNA-treated populations are represented by light gray bars. Dark gray bars represent control populations.
Figure 2.
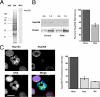
Cells containing multiple micronuclei are depleted of Nup358. A single high molecular weight band is recognized on a Western blot of a HeLa whole cell extract by the anti-Nup358 antibodies that are employed in this study (A). Total protein content of the cell extract is shown in the Coomassie blue–stained lane (gel). Molecular weight markers (MW, kD) are indicated on the left. A 50% reduction in total Nup358 levels in Nup358 siRNA–treated (RNAi) versus control cultures (Mock) is shown by quantitative Western blot analysis (B). For this experiment, twofold dilution series of HeLa cell extracts were analyzed by Western blot using antibodies against Nup358 (A) as well as β-tubulin as an internal standard. Two sample pairs (differing in concentration by a factor of two) from both control and siRNA-treated cultures are shown. Changes in Nup358 levels were determined from the ratios of the Nup358/β-tubulin band intensities. These experiments were performed in quadruplicate over a 16-fold sample concentration range. Immunofluorescence microscopy of Nup358 siRNA–treated cultures reveals a loss of NE-associated Nup358 (C). This is particularly evident in cells containing multiple micronuclei. A second nucleoporin, Nup153, shows no such decline. Fluorescence intensity measurements performed on randomly selected interphase cells (Total) indicate a 66% reduction in Nup358 levels (±5%; P < 0.001) in siRNA-treated versus control (Mock) cells. A larger average reduction in anti-Nup358 fluorescence intensity of 77% (±1%; P < 0.001) is observed when measurements are restricted to cells containing multiple micronuclei (MN). Bar, 10 μm.
Figure 3.
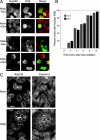
Cells depleted of Nup358 contain identifiable NPCs. Indirect immunofluorescence microscopy reveals little or no change in the levels or localizations of multiple nucleoporins, including Nup153 and Nup214, in multinucleate cells depleted of Nup358 (A and B). EM reveals the presence of abundant NPCs associated with micronuclei in cells subjected to Nup358 siRNA treatment (C). Immunofluorescence microscopy reveals that such cells are always depleted of Nup358 (D). Cells depleted of Nup358, including multinucleate cells, continue to incorporate A-type lamins (D) into the nuclear lamina over a period of 96 h. Bars: (A, B, and D) 10 μm; (C) 200 nm.
Figure 4.
Nuclei in cells depleted of Nup358 are import competent. Nuclear import of GRβ is unaffected in Nup358 siRNA–treated (RNAi) versus mock-treated cells. In the absence of dexamethasone (−Dex), GRβ remains almost exclusively cytoplasmic (A). After exposure to dexamethasone for 10 min (+Dex), GRβ relocates to the nucleus (or micronuclei). To establish a time course of GRβ import (B), cells were scored for nuclear accumulation of GRβ. This was defined as average nuclear fluorescence intensity greater than average cytoplasmic intensity. The graph (B) represents the mean of four experiments in which 200 cells were scored at each time point. For the RNAi samples, only cells clearly depleted of Nup358 were included in the counts. The overall distribution of the nuclear import receptor importin-β is also largely unaffected by depletion of Nup358 (C). Compare the large multinucleate cell in the RNAi panels with the mock-treated cells. Bars, 10 μm.
Figure 5.
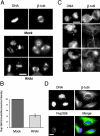
Abnormal chromosome congression and defective mitosis after Nup358 siRNA treatment. Indirect immunofluorescence microscopy of Nup358 siRNA–treated (RNAi) versus mock-treated HeLa cells. Prometaphase and metaphase cells labeled with anti–β-tubulin and Hoechst dye to reveal the condensed chromosomes (A). Up to 75% of premetaphase cells in siRNA-treated cultures exhibit abnormal or elongated spindle morphology associated with abnormal chromosome congression (A) featuring irregular chromosome clusters at the spindle equator as well as smaller clusters over each pole. This unusual morphology is rarely observed in control cells. Fluorescence intensity measurements on prometaphase cells labeled with an anti-Nup358 antibody (B) reveal an average reduction of 61% when compared with mock-treated cells (±6%; P < 0.001). Telophase and early G1 siRNA-treated cells exhibit a high frequency of multiple micronuclei and abnormal cytokinesis (C) in which lagging chromosomes and chromatin bridges connecting the daughters are observed (D).
Figure 6.
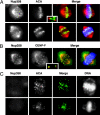
Loss of kinetochore-associated Nup358 after siRNA treatment. Double indirect immunofluorescence microscopy of mitotic HeLa cells using antibodies against Nup358 in combination either with a human anticentromere autoimmune serum (ACA) or an antibody against CENP-F. Cells were also labeled with Hoechst dye (shown in blue in A and B) to reveal the mitotic chromosomes. Untreated HeLa cells are shown in A and B. The insets reveal that Nup358 lies distal to the centromere and instead largely colocalizes with CENP-F, an outer kinetochore protein. Nup358 siRNA–treated cells are shown in C and reveal a loss of both kinetochore and spindle-associated Nup358, although ACA labeling is unaffected. Chromosome congression is clearly abnormal in these cells. In A and B, merged images are shown both with (right) and without (left) superimposition of the chromosomes. In C, the merged images show only the antibody labeling. In each set, Nup358 labeling is shown in red.
Figure 7.
Nup358 siRNAi treatment causes changes in both kinetochore composition and morphology in prometaphase cells. Double indirect immunofluorescence microscopy of mitotic HeLa cells reveals that multiple proteins are lost from kinetochores in Nup358 siRNA– versus mock-treated cells (A). These include CENP-E, CENP-F, dynein, and Mad1 (A) as well as Mad2 and Zw10 (B; siRNA-treated cell populations are represented by light gray bars, whereas dark gray indicates control populations). Occasional kinetochore labeling is observed with antibodies against these various components (A). This is invariably associated with polar chromosome clusters and not with the larger equatorial clusters. This is particularly evident in the CENP-E and CENP-F panels (A). Electron micrographs (C) of the kinetochores of control (a) versus siRNA-treated cells (b–d). The square brackets indicate a normal kinetochore with associated microtubules in panel a and expanded, diffuse kinetochores in b and c. C-shaped everted kinetochores are also a feature of Nup358 siRNA treatment (d, arrowheads). Bar, 600 nm.
Figure 8.
Depletion of Mad1 reverses the Nup358 siRNA–induced prometaphase arrest. Double indirect immunofluorescence microscopy of HeLa cell cultures treated with Nup358 and/or Mad1 siRNAs and labeled with antibodies against both Nup358 and Mad1 (A). In inter phase cells, Mad1, like Nup358, localizes almost exclusively to the nuclear periphery. Depletion of either protein in interphase cells has little or no effect on the distribution of the other. During mitosis, Nup358 depletion results in a significant (70%) increase in the number of premetaphase and metaphase cells over mock-treated cultures (B). This effect is reversed by codepletion of Mad1. Conversely, depletion of both proteins causes an increase in the number of multinucleate cells. 2–4,000 cells were scored in each category in four independent experiments. It should be noted that these counts represent percentages derived from total cell populations. To eliminate bias, no attempt was made to discriminate between transfected versus nontransfected cells. Bar, 10 μm.
Similar articles
- A role for gp210 in mitotic nuclear-envelope breakdown.
Galy V, Antonin W, Jaedicke A, Sachse M, Santarella R, Haselmann U, Mattaj I. Galy V, et al. J Cell Sci. 2008 Feb 1;121(Pt 3):317-28. doi: 10.1242/jcs.022525. J Cell Sci. 2008. PMID: 18216332 - Nucleophosmin is required for chromosome congression, proper mitotic spindle formation, and kinetochore-microtubule attachment in HeLa cells.
Amin MA, Matsunaga S, Uchiyama S, Fukui K. Amin MA, et al. FEBS Lett. 2008 Nov 12;582(27):3839-44. doi: 10.1016/j.febslet.2008.10.023. Epub 2008 Oct 23. FEBS Lett. 2008. PMID: 18951898 - RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment.
Raaijmakers JA, Tanenbaum ME, Maia AF, Medema RH. Raaijmakers JA, et al. J Cell Sci. 2009 Jul 15;122(Pt 14):2436-45. doi: 10.1242/jcs.051912. Epub 2009 Jun 23. J Cell Sci. 2009. PMID: 19549680 - [The relationship between nuclear-cytoplasmic transport and chromosome segregation].
Mamon LA. Mamon LA. Tsitologiia. 2005;47(3):263-76. Tsitologiia. 2005. PMID: 16706172 Review. Russian. - From pore to kinetochore and back: regulating envelope assembly.
Roux KJ, Burke B. Roux KJ, et al. Dev Cell. 2006 Sep;11(3):276-8. doi: 10.1016/j.devcel.2006.08.005. Dev Cell. 2006. PMID: 16950119 Review.
Cited by
- Aurora B SUMOylation Is Restricted to Centromeres in Early Mitosis and Requires RANBP2.
Di Cesare E, Moroni S, Bartoli J, Damizia M, Giubettini M, Koerner C, Krenn V, Musacchio A, Lavia P. Di Cesare E, et al. Cells. 2023 Jan 19;12(3):372. doi: 10.3390/cells12030372. Cells. 2023. PMID: 36766713 Free PMC article. - Mitotic spindle proteomics in Chinese hamster ovary cells.
Bonner MK, Poole DS, Xu T, Sarkeshik A, Yates JR 3rd, Skop AR. Bonner MK, et al. PLoS One. 2011;6(5):e20489. doi: 10.1371/journal.pone.0020489. Epub 2011 May 27. PLoS One. 2011. PMID: 21647379 Free PMC article. - Meiosis-specific loading of the centromere-specific histone CENH3 in Arabidopsis thaliana.
Ravi M, Shibata F, Ramahi JS, Nagaki K, Chen C, Murata M, Chan SW. Ravi M, et al. PLoS Genet. 2011 Jun;7(6):e1002121. doi: 10.1371/journal.pgen.1002121. Epub 2011 Jun 9. PLoS Genet. 2011. PMID: 21695238 Free PMC article. - Down-modulation of nucleoporin RanBP2/Nup358 impaired chromosomal alignment and induced mitotic catastrophe.
Hashizume C, Kobayashi A, Wong RW. Hashizume C, et al. Cell Death Dis. 2013 Oct 10;4(10):e854. doi: 10.1038/cddis.2013.370. Cell Death Dis. 2013. PMID: 24113188 Free PMC article. - The nuclear export factor Xpo1p targets Mad1p to kinetochores in yeast.
Scott RJ, Cairo LV, Van de Vosse DW, Wozniak RW. Scott RJ, et al. J Cell Biol. 2009 Jan 12;184(1):21-9. doi: 10.1083/jcb.200804098. J Cell Biol. 2009. PMID: 19139260 Free PMC article.
References
- Arnaoutov, A., and M. Dasso. 2003. The Ran GTPase regulates kinetochore function. Dev. Cell. 5:99–111. - PubMed
- Beaudouin, J., D. Gerlich, N. Daigle, R. Eils, and J. Ellenberg. 2002. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 108:83–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous