Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1 - PubMed (original) (raw)
. 2003 Sep 15;162(6):1149-60.
doi: 10.1083/jcb.200305018. Epub 2003 Sep 8.
Daniela Salomon, Hadas Elhanany, Helena Sabanay, Brent Kiernan, Larysa Pevny, Colin L Stewart, Xiaorong Xu, Shing-Yan Chiu, Peter Shrager, Andrew J W Furley, Elior Peles
Affiliations
- PMID: 12963709
- PMCID: PMC2172860
- DOI: 10.1083/jcb.200305018
Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1
Sebastian Poliak et al. J Cell Biol. 2003.
Abstract
In myelinated axons, K+ channels are concealed under the myelin sheath in the juxtaparanodal region, where they are associated with Caspr2, a member of the neurexin superfamily. Deletion of Caspr2 in mice by gene targeting revealed that it is required to maintain K+ channels at this location. Furthermore, we show that the localization of Caspr2 and clustering of K+ channels at the juxtaparanodal region depends on the presence of TAG-1, an immunoglobulin-like cell adhesion molecule that binds Caspr2. These results demonstrate that Caspr2 and TAG-1 form a scaffold that is necessary to maintain K+ channels at the juxtaparanodal region, suggesting that axon-glia interactions mediated by these proteins allow myelinating glial cells to organize ion channels in the underlying axonal membrane.
Figures
Figure 1.
Generation of Caspr2-null mice. (A) Schematic map of a genomic DNA fragment containing the first exon of Caspr2 and flanking region, the targeting construct, and the resulting allele in which exon 1 was replaced by a neo gene. The expected size of the DNA fragment detected using the 3′ probe (black box) is labeled in red. (B) Genomic PCR analysis of the indicated animals using specific primer sets of exon 1 of Caspr2, neo, or a control CGT gene as indicated. (C) Southern blot analysis. Genomic DNA digested with HindIII and PvuII was hybridized to the 3′ probe. The expected 4.8-kb and/or 3.3-kb fragments were detected in wild-type (+/+), heterozygote (−/+), and homozygote (−/−) animals. (D) Western blot analysis. Brain lysates prepared from the indicated mice were subjected to immunoprecipitation (IP) and immunoblotting, or directly blotted (Total) using antibodies to Caspr2 or Caspr. (E) Teased sciatic nerves of adult wild-type (WT) or _Caspr2_-null (−/−) mice were double labeled using antibodies to Na+ channel (red) and Caspr2 (green). Bar, 10 μm.
Figure 2.
**Morphology of the nodal environs in Caspr2−/**− mice. EM pictures of cross (A and E) and longitudinal (B and F) sections of sciatic nerve from adult wild-type (+/+; A and B), or Caspr2-deficient (−/−; E and F) mice are shown. Red arrowheads mark the location of the juxtaparanodes. C, D, G, and H (+/+, C and D; −/−, G and H), show double-immunofluorescence staining of the nodal region using antibodies to Na+ channels (red) and Caspr (green; C and G) or to NF155 (green; D and H). Bars: A and E, 200 nm; B and F, 1 μm.
Figure 3.
Distribution of K + channels in the CNS. Sections of optic nerve from wild-type (A, C, E, G, I, and K) or _Caspr2_-null mice (B, D, F, H, J, and L) were double labeled with antibodies to Caspr (C, D, I, and J) and Kvβ2 (G and H), or Kv1.2 (A and B). Merge images are shown in panels E, F, K, and L. The images were obtained under the same exposure conditions. Note the decrease in K+ channels staining in _Caspr2_−/− nerves. Inset in L shows measurements of the fluorescence intensity labeling of K+ channels in _Caspr2_-null compared with wild-type nerves in integrated optical density units. Three images at 40× were used for each genotype, and errors are given as ± SEM. Inset in F shows the levels of Kv1.2 protein detected by immunoblots in sciatic nerve lysates from wild-type (+/+) or _Caspr2_-null (−/−) mice. Bar, 20 μm.
Figure 4.
Reduced juxtaparanodal accumulation of K + **channels in the PNS of Caspr2−/**− mice. (A–F) Images showing immunofluorescence staining of sciatic nerve sections from wild-type (A–C) or _Caspr2_-deficient mice (D–F), using antibodies to Kv1.2 (red; A and D) and Caspr (green; B and E) as indicated. Merge images are shown on the right (C and F). Representative images of the nodal region from teased fiber preparations are shown in the insets in C and F. (G and H) Teased sciatic nerves from wild-type (G) and _Caspr2_-null (H) mice, labeled with antibodies to Kv1.2 (green) and Caspr (red). (I) Quantification of the percentage of juxtaparanodes exhibiting normal appearance of Kv1.2, identified as the domain adjacent to Caspr-stained area (n = +/+ 174, −/− 219). (J) Immunolabeling of teased sciatic nerve from _Caspr2_−/− mutant, showing intense staining of Kv1.2 along the internodes, entering the juxtaparanodal region. (Inset) Western blot showing the expression of Kv1.2 in sciatic nerve lysates from wild-type (+/+) and _Caspr2_-null (−/−) mice. (K) Higher magnification of the labeled frame in J. (L) Another example of the localization of K+ channels in the mutant, present in a double line crossing the juxtaparanodal region and terminating as a ring at the border between the juxtaparanodes and paranodes. The location of the paranodes is marked (P) in K and L. Bars: A–H, 20 μm; J–L, 10 μm of the bar shown in J.
Figure 5.
Absence of TAG-1 at the juxtaparanode in Caspr2 PNS. Double-immunofluorescence staining of teased sciatic nerves isolated from wild-type (A–C) or Caspr2-null mice (D–F), using antibodies to TAG-1 (green; A and D) and Na+ channels (red; B and E). Merge images are shown on the right of each row (C and F). Bar, 20 μm.
Figure 6.
Generation of TAG-1–deficient mice. (A) Map of recombination strategy showing part of TAG-1 gene locus including exon 2, which encodes the ATG and signal sequence. Below is the tau-LacZ–containing targeting construct and the predicted locus after targeting. Red lines indicate HindIII fragments detected with 5′ probe (black box). Orange and blue arrows indicate PCR primers used in C and D, respectively. (B) Southern blot showing targeting of construct in ES cells with HindIII digest and 5′ probe. (C) Detection of targeted locus in wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice by PCR. Wild-type TAG-1 allele detected by orange primer pair (see A) gives and ∼450-bp product, whereas targeted allele detected by neo-specific primers gives an ∼260-bp product. (D) RT-PCR to detect TAG-1 mRNA in postnatal cerebellum using blue primer set (see A). The expected 255-bp product is detected in heterozygote but not homozygote animals. (E) Western blot of postnatal cerebellum lysates from heterozygote and homozygote mice blotted with anti-TAG-1 pAbs. TAG-1 protein is detected in heterozygote mice, but not in the mutant. (F) Double-immunofluorescence staining of teased sciatic nerves from adult wild-type (WT), or _TAG-1_–null (−/−) mice using antibodies to Na+ channel (red) and TAG-1 (green). Bar, 10 μm.
Figure 7.
Distribution of Caspr2 and K + channels in peripheral nerves from TAG-1–deficient mice. Teased sciatic nerve fibers from wild-type (+/+; A–C and G–I) or homozygous (−/−; D–F and J–L) mice were analyzed. (A–F) Double labeling was performed using antibodies to Caspr2 (A and D; green) and Na+ channels (NaCh; B and E; red). Merge images are shown on the right (C and F). Occasionally, some weak staining of Caspr2 could still be observed at the juxtaparanodes. Western blot showing the expression of Caspr2 in sciatic nerves from wild-type (+/+) and TAG-1–null mice (−/−) is presented in the inset of F. (G–L) Immunofluorescence staining of a similar preparation with antibodies to Kv1.2 (red; G and J) and Caspr (green; H and K). Inset in L depicts the levels of Kv1.2 protein in sciatic nerve lysates from wild-type (+/+) or _TAG-1_–null (−/−) mice. Bar, 20 μm.
Figure 8.
Association of Caspr2 with TAG-1. (A) Association of TAG-1 and Caspr2 in rat brain. Immunoprecipitation (IP) from rat brain membrane lysates was performed using antibodies to TAG-1 (IC12; TAG-1 mAb or rabbit pAb; TAG-1 pAb), Kv1.2, Kv2.1, contactin, or Caspr2 as indicated, followed by immunoblotting with an antibody to Caspr2. Anti-mouse (mBeads) or protein A (rBeads) beads were used as additional controls. (B) Co-immunoprecipitation of TAG-1 and K+ channels. Rat brain membrane lysates were subjected to immunoprecipitation using the indicated antibodies, followed by blotting with an antibody to Kv1.2. Total protein extract (Total) was used to determine the location of Kv1.2 on the gel. Note that Kv1.2 was detected using two different antibodies to TAG-1. (C) Association of TAG-1 and Caspr2 in transfected cells. Lysates of HEK-293 cells expressing Caspr2 and TAG-1, Caspr2 and contactin (CNTN), or Caspr and contactin (Cells), were used for immunoprecipitation (IP) and immunoblotting using different combinations of antibodies as indicated in each panel. Note that Caspr2 associated with TAG-1, but not with contactin, which interacts with Caspr. (D) Immunofluorescence staining showing surface expression of Caspr2. COS-7 cells expressing Caspr2 were stained using an antibody against its extracellular (ECD) or intracellular (CT) region, with or without permeabilization as indicated (−Tx, without Triton X-100; +Tx, with Triton X-100). Caspr2 immunoreactivity was detected using the ECD, but not CT antibody in nonpermeabilized cells. Bar, 50 μm. (E) TAG-1 binds homophilically, but not to Caspr2. A soluble TAG-1–Fc was allowed to bind COS-7 cells expressing TAG-1, Caspr2, Caspr2 and TAG-1, or contactin as indicated. Bound Fc fusion was detected using Cy3-conjugated anti-human Fc antibody (red). Note that TAG-1–Fc only bound to TAG-1– or TAG-1/Caspr2-expressing cells, but not to cells expressing Caspr2 or contactin. The insets on the top right of each panel show staining for the corresponding transfected proteins. Bar, 50 μm. (F) Top: β-galactosidase staining of adult sciatic nerve (SN) from heterozygous TAG-1_–_LacZ animals. Bottom: β-galactosidase staining of adult dorsal root ganglion (DRG). Intense lacZ expression was detected in cell bodies and the track (asterisk). Bars: D and E, 50 μm; F (top), 50 μm; F (bottom), 100 μm.
Figure 9.
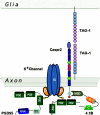
Schematic model describing molecular interactions at the juxtaparanodal region of myelinated axons. A cis complex of Caspr2 and TAG-1 is present at the axolemma. Homophilic interactions mediate the binding of this complex to glial TAG-1 present on the adaxonal membrane. The association of Caspr2 and K+ channels involves the PDZ-binding sequence of both proteins, and is likely mediated by yet unidentified PDZ domain–containing protein/s. The juxtaparanodes contain PSD-95, which binds K+ channels, but not Caspr2. The cytoplasmic region of Caspr2 also binds to protein 4.1B present at this site, which may connect the whole complex to the axonal cytoskeleton. PDZ, PSD-95/discs large/zona occludens 1; GUK, guanylate kinase; SH3, Src homology 3; MBD, membrane-binding domain.
Similar articles
- Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers.
Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B, Girault JA, Karagogeos D. Traka M, et al. J Cell Biol. 2003 Sep 15;162(6):1161-72. doi: 10.1083/jcb.200305078. J Cell Biol. 2003. PMID: 12975355 Free PMC article. - Localization of Caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon.
Poliak S, Gollan L, Salomon D, Berglund EO, Ohara R, Ranscht B, Peles E. Poliak S, et al. J Neurosci. 2001 Oct 1;21(19):7568-75. doi: 10.1523/JNEUROSCI.21-19-07568.2001. J Neurosci. 2001. PMID: 11567047 Free PMC article. - Caspr and caspr2 are required for both radial and longitudinal organization of myelinated axons.
Gordon A, Adamsky K, Vainshtein A, Frechter S, Dupree JL, Rosenbluth J, Peles E. Gordon A, et al. J Neurosci. 2014 Nov 5;34(45):14820-6. doi: 10.1523/JNEUROSCI.3369-14.2014. J Neurosci. 2014. PMID: 25378149 Free PMC article. - Glial regulation of the axonal membrane at nodes of Ranvier.
Schafer DP, Rasband MN. Schafer DP, et al. Curr Opin Neurobiol. 2006 Oct;16(5):508-14. doi: 10.1016/j.conb.2006.08.003. Epub 2006 Sep 1. Curr Opin Neurobiol. 2006. PMID: 16945520 Review. - The local differentiation of myelinated axons at nodes of Ranvier.
Poliak S, Peles E. Poliak S, et al. Nat Rev Neurosci. 2003 Dec;4(12):968-80. doi: 10.1038/nrn1253. Nat Rev Neurosci. 2003. PMID: 14682359 Review.
Cited by
- Loss of ASD-related molecule Cntnap2 affects colonic motility in mice.
Robinson BG, Oster BA, Robertson K, Kaltschmidt JA. Robinson BG, et al. Front Neurosci. 2023 Nov 9;17:1287057. doi: 10.3389/fnins.2023.1287057. eCollection 2023. Front Neurosci. 2023. PMID: 38027494 Free PMC article. - Antibody-Mediated Nodo- and Paranodopathies.
Quinot V, Rostasy K, Höftberger R. Quinot V, et al. J Clin Med. 2024 Sep 25;13(19):5721. doi: 10.3390/jcm13195721. J Clin Med. 2024. PMID: 39407781 Free PMC article. Review. - Dissecting the contribution of host genetics and the microbiome in complex behaviors.
Buffington SA, Dooling SW, Sgritta M, Noecker C, Murillo OD, Felice DF, Turnbaugh PJ, Costa-Mattioli M. Buffington SA, et al. Cell. 2021 Apr 1;184(7):1740-1756.e16. doi: 10.1016/j.cell.2021.02.009. Epub 2021 Mar 10. Cell. 2021. PMID: 33705688 Free PMC article. - Shining a light on CNTNAP2: complex functions to complex disorders.
Rodenas-Cuadrado P, Ho J, Vernes SC. Rodenas-Cuadrado P, et al. Eur J Hum Genet. 2014 Feb;22(2):171-8. doi: 10.1038/ejhg.2013.100. Epub 2013 May 29. Eur J Hum Genet. 2014. PMID: 23714751 Free PMC article. Review. - Compression induces acute demyelination and potassium channel exposure in spinal cord.
Ouyang H, Sun W, Fu Y, Li J, Cheng JX, Nauman E, Shi R. Ouyang H, et al. J Neurotrauma. 2010 Jun;27(6):1109-20. doi: 10.1089/neu.2010.1271. J Neurotrauma. 2010. PMID: 20373847 Free PMC article.
References
- Arroyo, E.J., and S.S. Scherer. 2000. On the molecular architecture of myelinated fibers. Histochem. Cell Biol. 113:1–18. - PubMed
- Arroyo, E.J., Y.T. Xu, L. Zhou, A. Messing, E. Peles, S.Y. Chiu, and S.S. Scherer. 1999. Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvbeta2, and Caspr. J. Neurocytol. 28:333–347. - PubMed
- Arroyo, E.J., T. Xu, S. Poliak, M. Watson, E. Peles, and S.S. Scherer. 2001. Internodal specializations of myelinated axons in the central nervous system. Cell Tissue Res. 305:53–66. - PubMed
- Baumgartner, S., J.T. Littleton, K. Broadie, M.A. Bhat, R. Harbecke, J.A. Lengyel, R. Chiquet-Ehrismann, A. Prokop, and H.J. Bellen. 1996. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 87:1059–1068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials