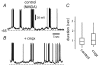Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice - PubMed (original) (raw)
Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice
R Vergara et al. J Physiol. 2003.
Abstract
In a rat corticostriatal slice, brief, suprathreshold, repetitive cortical stimulation evoked long-lasting plateau potentials in neostriatal neurons. Plateau potentials were often followed by spontaneous voltage transitions between two preferred membrane potentials. While the induction of plateau potentials was disrupted by non-NMDA and NMDA glutamate receptor antagonists, the maintenance of spontaneous voltage transitions was only blocked by NMDA receptor and L-type Ca2+ channel antagonists. The frequency and duration of depolarized events, resembling up-states described in vivo, were increased by NMDA and L-type Ca2+ channel agonists as well as by GABAA receptor and K+ channel antagonists. NMDA created a region of negative slope conductance and a positive slope crossing indicative of membrane bistability in the current-voltage relationship. NMDA-induced bistability was partially blocked by L-type Ca2+ channel antagonists. Although evoked by synaptic stimulation, plateau potentials and voltage oscillations could not be evoked by somatic current injection--suggesting a dendritic origin. These data show that NMDA and L-type Ca2+ conductances of spiny neurons are capable of rendering them bistable. This may help to support prolonged depolarizations and voltage oscillations under certain conditions.
Figures
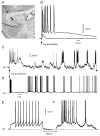
Figure 1. Cortical stimulation evoked long-lasting plateau potentials with repetitive firing in medium spiny neurons
A, scheme of a brain slice showing the connection stimulated. The stimulating electrode was placed in the cortical grey or white matter (pyramidal cell scheme), and the recording electrode on the dorsal neostriatum (neostriatal cell scheme). B, orthodromic response of a spiny neostriatal neuron to single subthreshold and suprathreshold stimulus in the cortex. Notice plateau potential with repetitive discharge after suprathreshold stimulus. C, a three times threshold cortical stimulus now evokes a plateau potential followed by several membrane potential oscillations, some of which exhibit the firing of action potentials. This behaviour is more commonly elicited with a stimulus train (see Fig. 4_B_). The time scale is different in B and C. D, a stimulus train can also evoke an arrhythmic sequence of voltage oscillations in pyramidal cells (sensorimotor cortex, layer 5). E, firing of a neostriatal neuron after a intracellular current injection at the soma (current stimulus not shown). F, firing of a neostriatal neuron during an spontaneous depolarization or ‘up-state’. Action potentials are clipped due to digitization procedures in E and F.
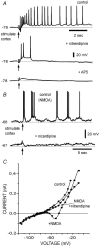
Figure 4. NMDA facilitates plateau potentials and membrane potential oscillations
A, a pair of synaptic responses was evoked with cortex stimulation. Only the second EPSP reached threshold (control). Addition of NMDA (25 µ
m
) increased the duration of the second response and produced a plateau potential. Thus, NMDA mimicked an increase in stimulus strength (+NMDA). B, a train of orthodromic subthreshold stimuli were given at the cortex (arrow signals upward deflections of stimulus artifacts). The response was subthreshold to produce any plateau or oscillatory response (control). Superfusion with NMDA (25 µ
m
) without a cortical stimulus did not produce oscillatory responses in most cases (+NMDA). However, the combined administration of a cortical stimulus and NMDA evoked membrane potential oscillations after a variable latency (two continuous traces at bottom). C, a 2 s ramp was given at the soma and the whole current response plotted against this voltage command. Control response shows firing of unclamped action currents but no crossings of the zero current axis. Addition of NMDA (20 µ
m
) facilitated a negative slope conductance region, and a double crossing of the zero current axis, indicative of bistability (+NMDA). D, reduction of the electrotonic length with Ba2+, shifted the first crossing of the zero-current axis and the negative slope conductance region to the left in the voltage axis. This suggests that the origin of bistability is located on the dendrites. The graph also shows the inward rectifier block by Ba2+.
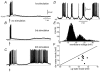
Figure 2. Evoked oscillation of the membrane potential after repetitive cortical stimulation
A, a pair of cortical stimuli (arrow) produces a plateau potential similar to that depicted in Fig. 1 (cf. time scale). B, repetition of the stimulus at 0.1 Hz produced a late subthreshold depolarization after the plateau potential produced by the third stimulus. C, continuous repetition of the same stimulus induced a suprathreshold late depolarization after the plateau potential evoked by the sixth stimulus. D, a continuous oscillation of the membrane potential followed this later plateau (see change in time scale, action potentials are clipped or incomplete due to digitization) without any further cortical stimulus. Several cells exhibited a tendency to slowly depolarize but continued to exhibit voltage oscillations for several minutes without overt cortical stimulation. E, an all point histogram of the membrane potential is shown for this cell. Notice Gaussian distributions around two different membrane potential values. F, a scatter plot for two-state membrane potential is shown for a sample of cells. This experiment was performed in the absence of NMDA. However, in other cells, NMDA greatly facilitated the appearance of membrane potential oscillations after the initial, evoked, plateau potential.
Figure 3. Plateau potentials are easily evoked after paired pulse stimulation
A, from top to bottom, the time interval between a pair of cortical stimuli was reduced without changing the stimulus strength. Notice a prolonged depolarization capable of sustaining repetitive firing after the briefer interval. B, the responses to a pair of subthreshold stimuli are shown. They were recorded at two different holding potentials. Paired-pulse facilitation is shown at −60 mV, and depression at −70 mV (top). However, if stimulus strength was increased, a prolonged depolarization could be elicited at −70 mV (bottom). This was never obtained by depolarizing the soma with current injections. C, a cell was hyperpolarized to −92 mV (top) while having voltage oscillations, as in Fig. 2_D_. Oscillations were reduced in both duration and amplitude to finally stop after this manoeuvre. An all point histogram (bottom) revealed bimodality of the membrane potential at −70 mV, but only a single peak at −92 mV.
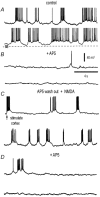
Figure 5. AP5 blocks membrane potential oscillations
A, representative sweeps of spontaneous (in the absence of cortical stimulus or NMDA) membrane potential oscillations recorded in a neostriatal neuron (control). Action potentials are clipped or incomplete due to digitizing procedures. B, addition of AP5 (50 µ
m
) in the bath saline blocked the membrane potential oscillations (+AP5). C, membrane potential oscillations could be restored after cortical stimulation and addition of NMDA (10 µ
m
) during AP5 wash-out (AP5 wash-out + NMDA). D, AP5 could block voltage oscillations induced by cortical stimulation and NMDA in a sample of cells as this one (+AP5 traces at bottom).
Figure 7. L-type Ca2+ channels participate in the generation of synaptically evoked plateau potentials and membrane potential oscillations
A, a pair of suprathreshold cortical stimuli evoked a long-lasting plateau potential in a spiny cell (control). Notice sustained firing. Addition of the L-type Ca2+ channel antagonist, nitrendipine (5 µ
m
), blocked most of the plateau potential (+nitrendipine). The subsequent addition of the NMDA receptor antagonist, AP5 (50 µ
m
), blocked the remaining slow depolarization disclosing the underlying EPSPs (+AP5). B, membrane potential oscillations were evoked with cortical stimulation and NMDA (10 µ
m
)(control). Addition of the L-type Ca2+ channel antagonist, nicardipine (5 µ
m
), blocked the oscillations and made cortical stimulation ineffective for evoking them. C, superimposed current–voltage (I–V) plots from a medium spiny cell (control). Addition of NMDA (20 µ
m
) induces a negative slope conductance region and bistability. A subsequent addition of nitrendipine (5 µ
m
) to the bath saline abolished the negative slope conductance region induced by NMDA.
Figure 6. CNQX does not block membrane potential oscillations
A, membrane potential oscillations were induced by repetitive cortical stimulation plus NMDA (10 µ
m
). B, once induced, membrane potential oscillations did not change in frequency or duration after the addition of CNQX (10 µ
m
). C, box plots illustrate distributions of up-state duration before and during CNQX in a sample of neurons (n = 7).
Figure 8. L-type Ca2+ channel agonist, BayK 8644, increased the duration of membrane potential oscillations
A, membrane potential oscillations were evoked by cortical stimulation plus NMDA (20 µ
m
; control). Addition of BayK 8644 (5 µ
m
) increased the duration of up-state events (+BayK 8644 and B). C, the amplitude of these events was also increased after BayK 8644 (C).
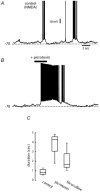
Figure 9. GABAA receptor antagonists enhance up-state duration
A, membrane potential oscillations were induced by repetitive cortical stimulation plus NMDA (10 µ
m
). B, the duration of up-states was enhanced by the addition of GABAA receptor antagonists to the bath saline (+picrotoxin, 5 µ
m
). C, box plots illustrate distributions of up-state duration before and during the GABAA receptor antagonists picrotoxin and bicuculline (5 µ
m
) in a sample of neurons (n = 6).
Similar articles
- Delayed postnatal development of NMDA receptor function in medium-sized neurons of the rat striatum.
Hurst RS, Cepeda C, Shumate LW, Levine MS. Hurst RS, et al. Dev Neurosci. 2001;23(2):122-34. doi: 10.1159/000048704. Dev Neurosci. 2001. PMID: 11509835 - Slow afterhyperpolarization governs the development of NMDA receptor-dependent afterdepolarization in CA1 pyramidal neurons during synaptic stimulation.
Wu WW, Chan CS, Disterhoft JF. Wu WW, et al. J Neurophysiol. 2004 Oct;92(4):2346-56. doi: 10.1152/jn.00977.2003. Epub 2004 Jun 9. J Neurophysiol. 2004. PMID: 15190096 - Synaptic activation of dendritic AMPA and NMDA receptors generates transient high-frequency firing in substantia nigra dopamine neurons in vitro.
Blythe SN, Atherton JF, Bevan MD. Blythe SN, et al. J Neurophysiol. 2007 Apr;97(4):2837-50. doi: 10.1152/jn.01157.2006. Epub 2007 Jan 24. J Neurophysiol. 2007. PMID: 17251363 - Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum.
Cepeda C, Levine MS. Cepeda C, et al. Dev Neurosci. 1998;20(1):1-18. doi: 10.1159/000017294. Dev Neurosci. 1998. PMID: 9600386 Review. - The generation of natural firing patterns in neostriatal neurons.
Wilson CJ. Wilson CJ. Prog Brain Res. 1993;99:277-97. doi: 10.1016/s0079-6123(08)61352-7. Prog Brain Res. 1993. PMID: 8108553 Review. No abstract available.
Cited by
- A Novel De Novo Gain-of-Function CACNA1D Variant in Neurodevelopmental Disease With Congenital Tremor, Seizures, and Hypotonia.
Dannenberg F, Von Moers A, Bittigau P, Lange J, Wiegand S, Török F, Stölting G, Striessnig J, Motazacker MM, Broekema MF, Schuelke M, Kaindl AM, Scholl UI, Ortner NJ. Dannenberg F, et al. Neurol Genet. 2024 Sep 6;10(5):e200186. doi: 10.1212/NXG.0000000000200186. eCollection 2024 Oct. Neurol Genet. 2024. PMID: 39246741 Free PMC article. - Rhythmic activity in a forebrain vocal control nucleus in vitro.
Solis MM, Perkel DJ. Solis MM, et al. J Neurosci. 2005 Mar 16;25(11):2811-22. doi: 10.1523/JNEUROSCI.5285-04.2005. J Neurosci. 2005. PMID: 15772341 Free PMC article. - Dopaminergic modulation of striatal plateau depolarizations in corticostriatal organotypic cocultures.
Tseng KY, Snyder-Keller A, O'Donnell P. Tseng KY, et al. Psychopharmacology (Berl). 2007 Apr;191(3):627-40. doi: 10.1007/s00213-006-0439-7. Epub 2006 Jun 7. Psychopharmacology (Berl). 2007. PMID: 16758237 Free PMC article. - Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons.
Carter AG, Soler-Llavina GJ, Sabatini BL. Carter AG, et al. J Neurosci. 2007 Aug 15;27(33):8967-77. doi: 10.1523/JNEUROSCI.2798-07.2007. J Neurosci. 2007. PMID: 17699678 Free PMC article. - Duration differences of corticostriatal responses in striatal projection neurons depend on calcium activated potassium currents.
Arias-García MA, Tapia D, Flores-Barrera E, Pérez-Ortega JE, Bargas J, Galarraga E. Arias-García MA, et al. Front Syst Neurosci. 2013 Oct 4;7:63. doi: 10.3389/fnsys.2013.00063. eCollection 2013. Front Syst Neurosci. 2013. PMID: 24109439 Free PMC article.
References
- Akopian G, Walsh JP. Corticostriatal paired-pulse potentiation produced by voltage-dependent activation of NMDA receptors and L-type Ca2+ channels. J Neurophysiol. 2002;87:157–165. - PubMed
- Bargas J, Ayala GX, Vilchis C, Pineda JC, Galarraga E. Ca2+-activated outward currents in neostriatal neurons. Neuroscience. 1999;88:429–488. - PubMed
- Bargas J, Galarraga E, Aceves J. Dendritic activity on neostriatal neurons as inferred from somatic intracellular recordings. Brain Res. 1991;539:159–163. - PubMed
- Beiser DG, Houk JC. Model of cortical-basal ganglionic processing: encoding the serial order of sensory events. J Neurophysiol. 1998;79:3168–3188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS034696/NS/NINDS NIH HHS/United States
- R01 DA012958/DA/NIDA NIH HHS/United States
- NS34696/NS/NINDS NIH HHS/United States
- TWO1214/TW/FIC NIH HHS/United States
- R37 NS034696/NS/NINDS NIH HHS/United States
- DA12958/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous