In vitro generation of early-born neurons from late retinal progenitors - PubMed (original) (raw)
In vitro generation of early-born neurons from late retinal progenitors
Jackson James et al. J Neurosci. 2003.
Abstract
Evidence suggests that, as development ensues, the competence of neural progenitors is progressively altered, such that they become fated to give rise to neurons of a particular stage. Here, we demonstrate that late retinal progenitors can give rise to retinal ganglion cells (RGCs), an example of an early-born cell type in the retina. A subset of late retinal progenitors in vitro responds to cues that favor RGC differentiation by displaying markers characteristic of RGCs. In addition, mechanisms used during normal RGC differentiation are recruited by these cells toward their differentiation along RGC lineage. Our observations suggest that late neural progenitors may not be irreversibly fated but may appear as such under the constraints dictated by epigenetic cues.
Figures
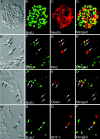
Figure 1.
Cultured retinal progenitors express late-born neuron- and RGC-specific markers. E18 retinal cells were cultured in the presence of EGF to enrich retinal progenitors as neurospheric cells that incorporated BrdU and expressed Nestin (A-D). Substitution of mitogen with 1% FBS led neurospheric cells to express PKC (E-H, arrows) and opsin (I-L, arrows), markers that characterize late-born neurons, bipolar cells, and rod photoreceptors, respectively. A small subset of cells (arrow) was observed in the presence of FBS that expressed RPF1, a marker expressed by early-born neurons, RGCs (M-P). Scale bar, 100 μm.
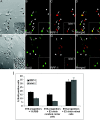
Figure 2.
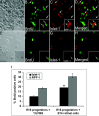
Differentiation of the late retinal progenitor into RGC is influenced by factor(s) elaborated by cells representing the early stage of histogenesis. Late retinal progenitors were tagged with BrdU and cultured with chick retinal cells harvested from stage E3. For controls, BrdU-tagged late retinal progenitors were cultured with E3 chick cerebral cortex cells. More BrdU-tagged late retinal progenitors (arrows) expressed RGC-specific markers, RPF1 and Islet1 (inset), in the presence of E3 chick retinal cells (A-D, arrowhead) than in the presence of cerebral cortex cells (E-H, arrowhead). The graph (I) depicts that the proportion of BrdU-tagged late retinal progenitors expressing RPF1 and Islet1 increased more significantly in the presence of E3 chick retinal cells than in the presence of 1% FBS (*RPF1,p< 0.05; **Islet1, p < 0.01) or E3 chick cerebral cortex cells (*RPF1, p < 0.01; **Islet1, p < 0.001). Values are means ± SEM of four to six separate observations, as assessed by one-way ANOVA. Scale bar, 100 μm.
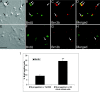
Figure 3.
Late retinal progenitors cocultured with E3 chick retinal dissociates express RGC-specific marker Brn3b. Late retinal progenitors tagged with BrdU were cocultured with E3 chick retinal dissociates (A-D). For controls, BrdU-tagged cells were cultured in the presence of 1% FBS (E-H). BrdU-tagged late retinal progenitors cocultured with E3 chick retinal cells expressed the RGC-specific marker Brn3b in a higher proportion compared with control. Graph (I) depicts significant increase in Brn3b under coculture condition compared with control (***p < 0.001). Values are means ± SEM of four to six separate observations, as assessed by Student's t test. Scale bar, 100 μm.
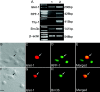
Figure 4.
Diffusible factors secreted by E3 chick retinal dissociate influence late retinal progenitors to express multiple RGC-specific transcripts. Immunocytochemical results were corroborated by RT-PCR analysis in late retinal progenitors (A). The levels (Islet1, RPF1, Thy1,and Brn3b) increased in late retinal progenitors cocultured with E3 chick retinal cells across a membrane (lane 2) compared with those cultured in the presence of 1% FBS (lane 1). Culture of late retinal progenitors with E3 chick-conditioned medium influenced late retinal progenitors to coexpress multiple RGC markers such as Islet1 and RPF1 (B-E, arrow), and Islet1 and Brn3b (F-I, arrow). Scale bar, 20 μm.
Figure 5.
Factors that promote differentiation of late retinal progenitors into RGCs are evolutionarily conserved. To determine the conserved nature of the epigenetic cues that promote RGC differentiation, BrdU-tagged late retinal progenitors were cocultured with rat retinal cells from the E14 stage of RGC genesis. Control included culturing progenitors in the presence of 1% FBS. More BrdU-tagged late retinal progenitors (arrows) expressed Islet1 and RPF1 (inset) in the presence of E14 retinal cells (A-D) than in the control (E-H). The graph (I) shows that the proportion of late retinal progenitors expressing Islet1 and RPF1 increased more significantly in the presence of E14 rat retinal cells than in the control (**Islet1, p < 0.01; *RPF1, p < 0.05), suggesting that RGC-promoting factors are conserved between chick and rat. Values are means ± SEM of four to six separate observations, as assessed by Student's t test. Scale bar, 100 μm.
Figure 6.
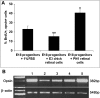
Factor(s) elaborated by cells representing the early stage of retinal histogenesis inhibit differentiation of late-born neurons. BrdU-tagged late retinal progenitors were cultured in the presence of 1% FBS, E3 chick retinal cells, and PN1 rat retinal cells for 5-7 d, and examined for the expression of opsin, a rod photoreceptor marker. The proportion of BrdU-tagged progenitors expressing opsin decreased significantly in the presence of E3 chick retinal cells compared with those in the presence of 1% FBS (**p < 0.01) (A). In contrast, the proportion of opsin-positive cells increased significantly when progenitors were cultured with PN1 retinal cells that have been shown to elaborate rod-promoting factors (*p < 0.01) (A). Values are means ± SEM of four to six separate observations, as assessed by one-way ANOVA. RT-PCR analysis of transcripts corresponding to opsin was performed on late retinal progenitors cultured with different embryonic cells across the membrane (B). The analysis showed that, compared with FBS control (lane 1), the level of opsin transcripts decreased in the presence of E3 chick retinal cells (lane 3), E3 chick cerebral cortex (lane 2), and PN1 cerebral cortex cells (lane 4), and increased in the presence of PN1 retinal cells (lane 5).
Figure 7.
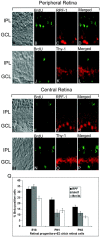
_In vitro_-generated RGCs are not derived from residual early retinal progenitors. Retinas of PN1 pups of pregnant rats, treated with BrdU at the gestation age of E18, were processed for immunocytochemical analysis using anti-BrdU antibodies and antibodies against RGC markers RPF1 (A-H) and Thy1 (I-P). BrdU-positive cells can be observed, but none of those coexpressed RPF1 or Thy1, in either the peripheral or central retina. Coculture of E18, PN1, and PN3 retinal progenitors with E3 chick retinal cells shows a progressive decrease in the cells expressing RGC-specific markers RPF1, Islet1, and Brn3b with each subsequent time point (Q). Values are means ± SEM of four to six separate observations, as assessed by one-way ANOVA. Scale bar, 50 μm. IPL, Inner plexiform layer; GCL, ganglion cell layer.
Figure 8.
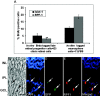
Late retinal progenitors are constrained from differentiating into RGC in vivo. Late retinal progenitors, tagged with BrdU in vivo at E18, were harvested and cultured immediately with E3 chick retinal cells for 5 d. Late retinal progenitors, tagged with BrdU in vitro after 7 d in the presence of mitogens, were cultured in the presence of 1% FBS for 5 d. At the end of culture, the proportion of BrdU-positive cells expressing Islet1 and RPF1 were examined (A). The proportion of in vivo BrdU-tagged cells expressing RPF1 and Islet1 was significantly lower than that of in vitro BrdU-tagged cells expressing RGC-specific markers (*Islet1, p < 0.05; **RPF1, p < 0.001). Values are means ± SEM of four to six separate observations, as assessed by Student's t test. GFP-expressing late retinal progenitors were transplanted intravitreally into the eyes of PN10 transgenic (P23H) rats. GFP-positive cells integrated into the host laminar structure of the retina and expressed RPF1, suggesting their differentiation along RGC lineage (B-F). Arrows indicate transplanted GFP + RPF1-positive cells. Scale bar, 50 μm. DAPI, 4′,6′-Diamidino-2-phenylindole; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer.
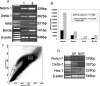
Figure 9.
Differentiation of late retinal progenitors into RGC involves Notch-1 receptor and bHLH transcription factor Ath5. RT-PCR analysis was performed on late retinal progenitors cultured in the presence of 1% FBS and E3 chick retinal cells to examine the levels of specific transcripts. The levels of transcripts corresponding to Notch-1, Delta-1, Ath5, and Brn3b were increased in coculture condition (lane 2) in comparison with that in 1% FBS control (lane 1) (A, B). Hoechst dye efflux assay was used to separate progenitor and precursor populations as SP and NSP, respectively (C). RT-PCR analysis performed on these two populations showed that levels of transcripts corresponding to Notch-1, Hes1, and Delta-1 were relatively higher in SP cells than in NSP cells (D).
References
- Ahmad I ( 1995) Mash-1 is expressed during ROD photoreceptor differentiation and binds an E-box, E(opsin)-1 in the rat opsin gene. Brain Res Dev Brain Res 90 : 184-189. - PubMed
- Ahmad I, Zaqouras P, Artavanis-Tsakonas S ( 1995) Involvement of Notch-1 in mammalian retinal neurogenesis: association of Notch-1 activity with both immature and terminally differentiated cells. Mech Dev 53 : 73-85. - PubMed
- Ahmad I, Dooley CM, Polk DL ( 1997) Delta-1 is a regulator of neurogenesis in the vertebrate retina. Dev Biol 185 : 92-103. - PubMed
- Ahmad I, Dooley CM, Afiat S ( 1998a) Involvement of Mash1 in EGF-mediated regulation of differentiation in the vertebrate retina. Dev Biol 194 : 86-98. - PubMed
- Ahmad I, Acharya HR, Rogers JA, Shibata A, Smithgall TE, Dooley CM ( 1998b) The role of NeuroD as a differentiation factor in the mammalian retina. J Mol Neurosci 11 : 165-178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical