Cell type-specific differences in chloride-regulatory mechanisms and GABA(A) receptor-mediated inhibition in rat substantia nigra - PubMed (original) (raw)
Cell type-specific differences in chloride-regulatory mechanisms and GABA(A) receptor-mediated inhibition in rat substantia nigra
Alexandra Gulácsi et al. J Neurosci. 2003.
Abstract
The regulation of intracellular chloride has important roles in neuronal function, especially by setting the magnitude and direction of the Cl- flux gated by GABA(A) receptors. Previous studies have shown that GABA(A)-mediated inhibition is less effective in dopaminergic than in GABAergic neurons in substantia nigra. We studied whether this phenomenon may be related to a difference in Cl-regulatory mechanisms. Light-microscopic immunocytochemistry revealed that the potassium-chloride cotransporter 2 (KCC2) was localized only in the dendrites of nondopaminergic (primarily GABAergic) neurons in the substantia nigra, whereas the voltage-sensitive chloride channel 2 (ClC-2) was observed only in the dopaminergic neurons of the pars compacta. Electron-microscopic immunogold labeling confirmed that KCC2 is localized in the dendritic plasma membrane of GABAergic neurons close to inhibitory synapses. Confocal microscopy showed that ClC-2 was selectively expressed in the somatic and dendritic cell membranes of the dopaminergic neurons. Gramicidin-perforated-patch recordings revealed that the GABA(A) IPSP reversal potential was significantly less negative and had a much smaller hyperpolarizing driving force in dopaminergic than in GABAergic neurons. The GABA(A) reversal potential was significantly less negative in bicarbonate-free buffer in dopaminergic but not in GABAergic neurons. The present study suggests that KCC2 is responsible for maintaining the low intracellular Cl- concentration in nigral GABAergic neurons, whereas a sodium-dependent anion (Cl--HCO3-) exchanger and ClC-2 are likely to serve this role in dopaminergic neurons. The relatively low efficacy of GABAA-mediated inhibition in nigral dopaminergic neurons compared with nigral GABAergic neurons may be related to their lack of KCC2.
Figures
Figure 1.
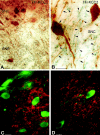
Light micrographs demonstrate the localization of KCC2 in nondopaminergic cells of the rat SN. A, B, Low (A)- and high (B)-magnification micrographs illustrate the segregation of TH-immunolabeled neurons (brown) and KCC2-immunopositive dendrites (blue-black) in both the SNc (SNC) and SNr (SNR). Both somata and dendrites of the dopaminergic neurons are labeled for TH (arrows). KCC-2 was found only in the dendritic compartment of nondopaminergic cells (arrowheads). C, D, Double-immunofluorescent staining shows a mutually exclusive distribution pattern for TH and KCC2. It demonstrates that none of the TH-positive (green) dendrites or somata are outlined by KCC2 immunoreactivity (red). Scale bars, 25 μm.
Figure 2.
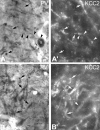
Conventional and epipolarization light micrographs show that, unlike dopaminergic cells, GABAergic neurons express KCC2 protein. A, Conventional light-microscopic image illustrates PV-immunopositive profiles in the rat SNr. PV exclusively labels a subset of GABAergic neurons in the SN. Black arrows indicate PV-immunopositive dendrites, whereas black arrowheads outline a PV-immunoreactive neuron with its labeled dendrite. _A_′, In the same section, epipolarization light microscopy illustrates KCC2-immunopositive dendrites. White arrowheads indicate a PV-immunopositive cell, of which only the dendrite but not the soma shows KCC2 immunoreactivity. White arrows show the PV-immunolabeled dendrites, which are also KCC2 immunoreactive. _B, B_′, Another example of conventional and epipolarization microscopy showing localization of KCC2 (_B_′**)** to PV+, nondopaminergic neurons (B). Scale bars, 20 μm. s, Soma.
Figure 3.
Electron-microscopic localization of KCC2 protein in the rat SN. A, B, Electron microscopy revealed that TH-immunopositive dendrites (DAB labeled) do not contain KCC2 protein (gold particles). Immunoreactivity for TH was found in dopaminergic dendrites, whereas KCC2 was localized in the dendritic membrane of nondopaminergic neurons. KCC2 immunolabeling (arrowheads) was often observed in the vicinity of symmetric synapses (arrows) at the postsynaptic site. Scale bars, 500 nm. b, Bouton; d, dendrite.
Figure 4.
Parvalbumin-immunoreactive neurons express KCC2 in the rat SN. A, B, Electron micrographs taken from two consecutive sections demonstrate the presence of KCC2 (gold particles) in the PV-immunopositive (DAB-labeled) dendrites. Gold particles show that KCC2 is often localized near to the symmetric synapses (arrows). Arrowheads indicate gold particles. Scale bar: (in A) A, B, 500 nm.
Figure 5.
Light-microscopic illustration of the distribution of ClC-2-immunolabeled neurons in the rat SN. A, Low-magnification light micrograph shows the presence of ClC-2-immunopositive cells in the SNc (SNC). ClC-2-immunopositive cells were visualized by immunoperoxidase reaction (DAB; black end product). The SNr (SNR) did not display ClC-2 immunoreactivity. B, High-magnification micrograph demonstrates that ClC-2 is present not only in the perikarya (arrowheads) but also in distal dendrites of the SNc neurons (arrows). Scale bars: A, 100 μm; B, 25 μm.
Figure 6.
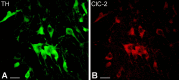
Confocal microscopic images of double-immunostained sections show that TH-immunoreactive dopaminergic neurons express ClC-2 in the SNc. A, Immunofluorescence staining against TH visualizes the dopaminergic neurons in the SNc (green). Both perikarya and dendrites display TH immunoreactivity. B, In the same section, ClC-2-immunopositive cells were identified on the basis of the presence of a red fluorescent signal. Similarly, immunolabeling was observed selectively in perikarya and proximal dendrites of dopaminergic neurons. Scale bars, 25 μm.
Figure 7.
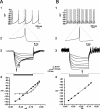
Identification of principal dopaminergic and GABAergic neurons in substantia nigra from current-clamp recordings. A1, A2, Dopaminergic neurons exhibit slow pacemaker-like firing with broad action potentials exceeding 2.5 msec in duration and a large-amplitude, long-duration spike afterhyperpolarization. A3, Dopaminergic neurons exhibit a prominent time-dependent sag in the voltage deflection in response to hyperpolarizing current injection because of a slowly activating _I_h. _A4, I_-V plots both before (filled circles) and during (open circles) activation of _I_h, calculated at the times indicated by the filled and open arrows in A3 above. The _I_-V relationship was best fit by linear regression before activation of _I_h but showed a pronounced inward rectification after its activation, which was best fit by a fourth-power polynomial. B1, B2, In contrast to dopaminergic neurons, pars reticulata principal GABAergic neurons fire spontaneously at higher rates, have a shorter-duration action potential of ∼1 msec in duration, and do not exhibit a prominent large-amplitude spike afterhyperpolarization. B3, B4, Principal GABAergic neurons lack _I_h, and the _I_-V plot, calculated at the time indicated by the arrow, is best fit by linear regression (B4). In both cases, the hyperpolarizing current was applied from rest. Traces in A3 and B3 are the digital averages of four consecutive single sweeps.
Figure 8.
Pharmacologically isolated GABAA receptor-mediated IPSPs in response to stimulation of the SNr in vitro. A, Representative current-clamp recordings of a dopaminergic neuron at various membrane potentials show an IPSP in response to stimulation of the SNr. Inset is a plot of the IPSP amplitude versus the membrane potential. The plot of the regression line revealed that the reversal potential of the IPSP was -62.9 mV. B, Representative current-clamp recordings of IPSP in GABAergic SNr neuron shows significantly more hyperpolarized reversal potential of -75.6 mV. C, D, In bicarbonate ion-free slice buffer, the GABAA receptor-mediated IPSP reversal potential in a representative dopaminergic neuron (C) becomes significantly less hyperpolarized at -49.3 mV, whereas there is no significant change in a nondopaminergic neuron in which the IPSP reverses at -67.7 mV (D). Each of the traces is an average of four sweeps.
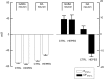
Figure 9.
A summary of values for the GABAA IPSP reversal potential (_E_IPSP-A) and the GABAA IPSP driving force (DFIPSP-A) in GABAergic and dopaminergic (DA) cells in standard (CTRL) and bicarbonate-free (HEPES) solution. _E_IPSP-A is significantly less hyperpolarized in dopaminergic than in GABAergic neurons under standard conditions. In the absence of bicarbonate, the _E_IPSP-A is significantly more positive, and the polarity of DFIPSP-A is reversed in the dopaminergic neurons. In contrast, there are no significant changes in these parameters in the GABAergic neurons.
Similar articles
- GABAergic afferents activate both GABAA and GABAB receptors in mouse substantia nigra dopaminergic neurons in vivo.
Brazhnik E, Shah F, Tepper JM. Brazhnik E, et al. J Neurosci. 2008 Oct 8;28(41):10386-98. doi: 10.1523/JNEUROSCI.2387-08.2008. J Neurosci. 2008. PMID: 18842898 Free PMC article. - Nigral GABAergic inhibition upon mesencephalic dopaminergic cell groups in rats.
Saitoh K, Isa T, Takakusaki K. Saitoh K, et al. Eur J Neurosci. 2004 May;19(9):2399-409. doi: 10.1111/j.0953-816X.2004.03337.x. Eur J Neurosci. 2004. PMID: 15128394 - GABAergic control of substantia nigra dopaminergic neurons.
Tepper JM, Lee CR. Tepper JM, et al. Prog Brain Res. 2007;160:189-208. doi: 10.1016/S0079-6123(06)60011-3. Prog Brain Res. 2007. PMID: 17499115 Review. - Subthalamic stimulation-induced synaptic responses in substantia nigra pars compacta dopaminergic neurons in vitro.
Iribe Y, Moore K, Pang KC, Tepper JM. Iribe Y, et al. J Neurophysiol. 1999 Aug;82(2):925-33. doi: 10.1152/jn.1999.82.2.925. J Neurophysiol. 1999. PMID: 10444687 - Double-edged GABAergic synaptic transmission in seizures: The importance of chloride plasticity.
Wang Y, Wang Y, Chen Z. Wang Y, et al. Brain Res. 2018 Dec 15;1701:126-136. doi: 10.1016/j.brainres.2018.09.008. Epub 2018 Sep 7. Brain Res. 2018. PMID: 30201259 Review.
Cited by
- A sensitive membrane-targeted biosensor for monitoring changes in intracellular chloride in neuronal processes.
Watts SD, Suchland KL, Amara SG, Ingram SL. Watts SD, et al. PLoS One. 2012;7(4):e35373. doi: 10.1371/journal.pone.0035373. Epub 2012 Apr 10. PLoS One. 2012. PMID: 22506078 Free PMC article. - Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons.
Henny P, Brown MT, Northrop A, Faunes M, Ungless MA, Magill PJ, Bolam JP. Henny P, et al. Nat Neurosci. 2012 Feb 12;15(4):613-9. doi: 10.1038/nn.3048. Nat Neurosci. 2012. PMID: 22327472 Free PMC article. - Evaluation of bumetanide as a potential therapeutic agent for Alzheimer's disease.
Boyarko B, Podvin S, Greenberg B, Momper JD, Huang Y, Gerwick WH, Bang AG, Quinti L, Griciuc A, Kim DY, Tanzi RE, Feldman HH, Hook V. Boyarko B, et al. Front Pharmacol. 2023 Aug 4;14:1190402. doi: 10.3389/fphar.2023.1190402. eCollection 2023. Front Pharmacol. 2023. PMID: 37601062 Free PMC article. Review. - Adolescent Nicotine Exposure Alters GABAA Receptor Signaling in the Ventral Tegmental Area and Increases Adult Ethanol Self-Administration.
Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, Brown-Mangum T, Dani JA. Thomas AM, et al. Cell Rep. 2018 Apr 3;23(1):68-77. doi: 10.1016/j.celrep.2018.03.030. Cell Rep. 2018. PMID: 29617674 Free PMC article. - Expression patterns of NKCC1 in neurons and non-neuronal cells during cortico-hippocampal development.
Kurki SN, Uvarov P, Pospelov AS, Trontti K, Hübner AK, Srinivasan R, Watanabe M, Hovatta I, Hübner CA, Kaila K, Virtanen MA. Kurki SN, et al. Cereb Cortex. 2023 May 9;33(10):5906-5923. doi: 10.1093/cercor/bhac470. Cereb Cortex. 2023. PMID: 36573432 Free PMC article.
References
- Celada P, Paladini CA, Tepper JM ( 1999) GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience 89 : 813-825. - PubMed
- Grace AA, Bunney BS ( 1979) Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur J Pharmacol 59 : 211-218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS34865/NS/NINDS NIH HHS/United States
- R01 NS034865/NS/NINDS NIH HHS/United States
- R56 NS034865/NS/NINDS NIH HHS/United States
- NS30549/NS/NINDS NIH HHS/United States
- R37 NS030549/NS/NINDS NIH HHS/United States
- R01 NS030549/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous