Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: a possible link to the microfilament system - PubMed (original) (raw)
Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: a possible link to the microfilament system
Torsten Giesemann et al. J Neurosci. 2003.
Abstract
Gephyrin is an essential component of the postsynaptic cortical protein network of inhibitory synapses. Gephyrin-based scaffolds participate in the assembly as well as the dynamics of receptor clusters by connecting the cytoplasmic domains of glycine and GABA(A) receptor polypeptides to two cytoskeletal systems, microtubules and microfilaments. Although there is evidence for a physical linkage between gephyrin and microtubules, the interaction between gephyrin and microfilaments is not well understood so far. Here, we show that neuronal gephyrin interacts directly with key regulators of microfilament dynamics, profilin I and neuronal profilin IIa, and with microfilament adaptors of the mammalian enabled (Mena)/vasodilator stimulated phosphoprotein (VASP) family, including neuronal Mena. Profilin and Mena/VASP coprecipitate with gephyrin from tissue and cells, and complex formation requires the E-domain of gephyrin, not the proline-rich central domain. Consequently, gephyrin is not a ligand for the proline-binding motif of profilins, as suspected previously. Instead, it competes with G-actin and phospholipids for the same binding site on profilin. Gephyrin, profilin, and Mena/VASP colocalize at synapses of rat spinal cord and cultivated neurons and in gephyrin clusters expressed in transfected cells. Thus, Mena/VASP and profilin can contribute to the postulated linkage between receptors, gephyrin scaffolds, and the microfilament system and may regulate the microfilament-dependent receptor packing density and dynamics at inhibitory synapses.
Figures
Figure 1.
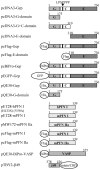
Prokaryotic and eukaryotic expression constructs and schematic representation of the encoded recombinant proteins: rat gephyrin (Gep) and its fragments, human profilin I (hPFN I), including mutants H133S and Y59A, respectively, murine profilins I (mPFN I) and IIa (mPFN IIa), VASP, and the β-subunit of the glycine receptor (β49). The diagram of the gephyrin polypeptide (Gep) organization shows the G-domain (G), the central domain (C) with the proline-rich sequence, and the E-domain (E) (Prior et al., 1992; Stallmeyer et al., 1999). Numbers refer to the amino acid residues. LPSPPPP, The proline-rich sequence within the central domain. The positions of the fused epitope tags (Flag, BiPro), the His6-tag, and the polypeptides GFP and intein with chitin binding domain (CBD) are indicated.
Figure 2.
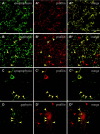
Profilin and gephyrin colocalize at rat spinal cord synaptic structures. Profilin was stained with a polyclonal antibody recognizing rodent profilins I and II. A, _A_″, Double immunofluorescence revealing synaptophysin, a synapse marker (A, green) with profilin (_A_′, red). The merged image (_A_″) shows that all synapses are enriched for profilin. B, _B_″, Corresponding double labeling for gephyrin, the postsynaptic marker of inhibitory neurons, with a monoclonal anti-gephyrin (B, green) and profilin (_B_′, red). This section was taken from a region of the spinal cord especially rich in inhibitory synapses. Note that all gephyrin-positive densities are also enriched in profilin (B, _B_″, yellow arrowheads), but many profilin-positive speckles (_B_′, _B_″, red arrowheads) lack gephyrin staining. C, _C_″, A cultured spinal cord neuron displaying numerous synaptophysin-positive dendritic synapses (C, green) that are also enriched for profilin (_C_′, red and merged image, _C_″, yellow, arrowheads). D, _D_″, Double immunofluorescence of gephyrin (D, green) and profilin (_D_′, red) of a cultured rat spinal cord neuron. Gephyrin and profilin show colocalization (D, _D_″, yellow arrowheads), but there are numerous profilin-positive densities not positive for gephyrin (_D_′, _D_″, red arrowheads). Scale bars, 10 μm.
Figure 3.
Gephyrin and profilins form complexes in cells and interact in vitro. A, _A_′, Affinity precipitation of profilin with gephyrin from rat (A) and mouse (_A_′) brain extracts, using beads coated with the gephyrin-binding sequence of the glycine receptor β-subunit (β49). Aliquots of extracts were incubated with beads, sedimented, and subjected to SDS-PAGE and immunoblotting, using polyclonal antibodies against gephyrin and both profilin isoforms. Lysate, Gephyrin and profilin shown in the total extracts; s:-β49 and p:-β49, supernatant and pellet of control beads coated only with the intein/chitin binding domain fusion peptide (see Material and Methods); s:+β49 and p:+β49, analogous probes obtained with beads coated with the gephyrin-binding sequence of the glycine receptor (β49). B, Cosedimentation of the β49 beads and recombinant profilin in the presence (+) or absence (-) of gephyrin. The pull-down assay was performed with recombinant gephyrin and recombinant profilin I, preincubated with an excess of poly-
l
-proline. SDS-PAGE and immunoblotting were as indicated in A and _A_′.
Figure 4.
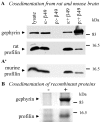
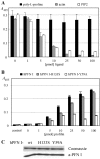
Profilin and gephyrin interact in brain tissue, cells, and in vitro. A, Immunoprecipitation from rat and mouse brain extracts, performed with the polyclonal anti-gephyrin (a-Gep). Sediments were subjected to SDS-PAGE and immunoblotting with a polyclonal anti-profilin. Control, Aliquot without anti-gephyrin. B, Immunoprecipitation of gephyrin and profilin from cell lysates. HeLa cells were transiently single transfected with BiPro-tagged gephyrin or double transfected with either Flag-tagged mouse profilin I or IIa, respectively. Immunoprecipitation was performed with the BiPro antibody, and immunoblots were obtained after SDS-PAGE. Transfected profilins were detected by Flag antibodies as indicated; endogenous profilin I was detected with a monoclonal profilin antibody. Note that endogenous human profilin I and both isoforms of transfected mouse profilins coimmunoprecipitated with transfected gephyrin. Control, Immunoblot derived from nontransfected HeLa cells. Lysates were treated with the BiPro antibody and protein G-Sepharose; sediments were subjected to SDS-PAGE and probed for endogenous profilin I. C, ELISA to show direct interaction between gephyrin and either profilin I or IIa. Twenty-five picomoles of recombinant gephyrin were adsorbed to ELISA wells and incubated with increasing amounts of recombinant profilin I (gray bars) and IIa (black bars) and a polyclonal anti-profilin recognizing both profilin isoforms. Note that both profilins bind to gephyrin with comparable affinity. Control, BSA (25 pmol) was immobilized and incubated with 100 pmol of mPFN I or mPFN IIa.
Figure 5.
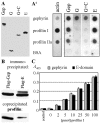
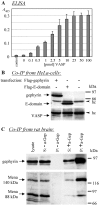
The E-domain of gephyrin interacts with profilins. A, _A_′, Overlay assays with in vitro translated, [35S]-Met-labeled proteins. Gep, Gephyrin; E, G, E+C, G+C, corresponding deletion fragments of gephyrin (cf. Fig. 1). A, Translation of polypeptides with the expected size was controlled by SDS-PAGE and autoradiography. _A_′, Binding of purified G-actin (left panel) and in vitro synthesized proteins (right panel, top) to membrane-adsorbed recombinant partners (left). BSA, Bovine serum albumin. Bound proteins were detected with specific antibodies (G-actin) or by autoradiography (others), respectively. Note that gephyrin interacts with itself and with both profilin isoforms and that binding is confined to the E-domain. B, Immunoblots obtained from HeLa cells transfected with Flag-tagged gephyrin or its E-domain. Immunoprecipitates were obtained with Flag antibody and probed with monoclonal anti-Flag and anti-profilin, demonstrating that the E-domain is sufficient to precipitate HeLa cell profilin I. C, ELISA with microwell-adsorbed recombinant gephyrin (gray bars) or the proteolytically derived E-domain (black bars; 25 pmol each) and soluble recombinant human profilin I. Binding of profilin was monitored with the monoclonal anti-profilin. Control was BSA (25 pmol) adsorbed to ELISA wells and incubated with 100 pmol of profilin. Note that in this assay the proteolytic fragment comprising the E-domain is as efficient in binding profilin as intact gephyrin.
Figure 6.
Gephyrin, PIP2, and G-actin compete for the same binding site on profilins. A, ELISAs demonstrating the effects of poly-
l
-proline, actin, or PIP2 on the interaction of human profilin I with gephyrin. Constant amounts of profilin I (25 pmol), preincubated with increasing amounts of poly-
l
-proline, actin, or PIP2 (picomole ligands), were added to ELISA plates coated with 25 pmol of gephyrin. Binding of profilin was monitored with the monoclonal anti-profilin. Note that preincubation of profilin with poly-
l
-proline had no significant effect on binding to gephyrin, whereas actin and PIP2 led to a dose-dependent reduction of profilin binding. Because PIP2 was added in the form of micelles, the effects of actin and PIP2 on profilin binding to gephyrin cannot be compared in terms of affinities. Error bars indicate SD of three independent experiments. B, ELISAs showing binding of gephyrin to mutant profilins. Microwell-adsorbed recombinant gephyrin was incubated with increasing amounts of recombinant wild-type human profilin I, or profilin with a point mutation in the poly-proline binding site (hPFN I-H133S) or in the actin binding site (hPFN I-Y59A), respectively. Binding was monitored with the monoclonal anti-profilin. Note that binding of hPFN I-H133S (gray bars) was decreased only slightly compared with hPFN I (black bars), whereas binding of hPFN I-Y59A (white bars) was significantly reduced. Control was 25 pmol of microwell-adsorbed BSA incubated with 100 pmol of the different profilins as indicated. C, Immunoblots of the profilin mutants and the same antibody used in B to show that the immunoreactivity of all samples used is comparable.
Figure 7.
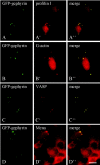
Profilin, G-actin, and Mena/VASP are enriched in gephyrin clusters. HeLa (_A_-_C, A_″-_C_″) and PC12 (_D, D_″) cells were transiently transfected with pEGFP-gephyrin resulting in the formation of large cytoplasmic gephyrin clusters (shown by green autofluorescence in _A_-D). _A, A_″, Cells cotransfected with pFlag-mPFN I. Immunofluorescence with the Flag antibody shows profilin I (_A_′, red) in the GFP-gephyrin aggregates. _B, B_″, Immunolabeling with an antibody specific for monomeric actin (_B_′, red) demonstrates endogenous nuclear actin as described (cf. Gonsior et al., 1999) and cytoplasmic G-actin in small cytoplasmic particles or dots and G-actin enrichment in each of the gephyrin clusters. _C, C_″, Endogenous VASP was detected with a monoclonal antibody (_C_′, red) as an additional component of gephyrin clusters in HeLa cells. _D, D_″, Immunofluorescence of GFP-gephyrin-transfected PC12 cells showing that gephyrin clusters in these paraneuronal cells are decorated with an antibody against the neuronal VASP relative Mena (_D_′, red). Colocalization is demonstrated by superimposition of the corresponding images (_A_″-_D_″). Scale bars, 10 μm.
Figure 8.
Gephyrin and Mena/VASP interact in vitro and form complexes in cells. A, ELISA to show direct interaction between gephyrin and VASP. Microwells were coated with 25 pmol of recombinant gephyrin and incubated with increasing amounts of recombinant BiPro-VASP as indicated. The BiPro-antibody was used to monitor binding. Note that binding of VASP to gephyrin reaches saturation level at a 1:1 molar ratio. Control was 25 pmol of BSA, microwell adsorbed, instead of gephyrin. B, Coimmunoprecipitation of VASP with gephyrin or the isolated E-domain. Immunoprecipitates obtained from HeLa cells transiently expressing Flag-tagged gephyrin or the Flag-tagged E-domain were obtained with the Flag antibody and subjected to SDS-PAGE and immunoblotting. Antibodies against Flag and VASP, respectively, revealed the transfected gephyrin or the E-domain and VASP in the precipitate, whereas VASP was not sedimented from nontransfected cells. Note that the E-domain of gephyrin is sufficient for binding to VASP. hc, Heavy chain of the precipitating IgG. C, Coimmunoprecipitation of Mena and gephyrin from rat brain extracts, performed with (+a-Gep) or without (-a-Gep) polyclonal anti-gephyrin. The immunoblots obtained with Mena antibodies from anti-gephyrin-induced precipitates reveal that two of the three Mena splice variants (88 and 140 kDa) are detectable in the sediment. Note that the large 140 kDa brain-specific isoform is preferentially enriched in the complex.
Figure 9.
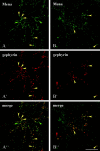
Colocalization of Mena with synaptic structures in cultured spinal cord neurons. Double immunofluorescence for Mena (A, B, green) and gephyrin (_A_′, _B_′, red). The merged images (_A_″, _B_″) show that most, but not all, synapses contain both proteins. Yellow arrowheads indicate examples of colocalization; green and red arrowheads indicate spots only positive for Mena and gephyrin, respectively. Scale bars, 10 μm.
Similar articles
- The state of the actin cytoskeleton determines its association with gephyrin: role of ena/VASP family members.
Bausen M, Fuhrmann JC, Betz H, O'sullivan GA. Bausen M, et al. Mol Cell Neurosci. 2006 Feb;31(2):376-86. doi: 10.1016/j.mcn.2005.11.004. Epub 2006 Jan 11. Mol Cell Neurosci. 2006. PMID: 16376568 - Interactions of drebrin and gephyrin with profilin.
Mammoto A, Sasaki T, Asakura T, Hotta I, Imamura H, Takahashi K, Matsuura Y, Shirao T, Takai Y. Mammoto A, et al. Biochem Biophys Res Commun. 1998 Feb 4;243(1):86-9. doi: 10.1006/bbrc.1997.8068. Biochem Biophys Res Commun. 1998. PMID: 9473484 - High-resolution structural analysis of mammalian profilin 2a complex formation with two physiological ligands: the formin homology 1 domain of mDia1 and the proline-rich domain of VASP.
Kursula P, Kursula I, Massimi M, Song YH, Downer J, Stanley WA, Witke W, Wilmanns M. Kursula P, et al. J Mol Biol. 2008 Jan 4;375(1):270-90. doi: 10.1016/j.jmb.2007.10.050. Epub 2007 Oct 24. J Mol Biol. 2008. PMID: 18001770 - The focal adhesion phosphoprotein, VASP.
Holt MR, Critchley DR, Brindle NP. Holt MR, et al. Int J Biochem Cell Biol. 1998 Mar;30(3):307-11. doi: 10.1016/s1357-2725(97)00101-5. Int J Biochem Cell Biol. 1998. PMID: 9611773 Review. - Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration.
Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Krause M, et al. Annu Rev Cell Dev Biol. 2003;19:541-64. doi: 10.1146/annurev.cellbio.19.050103.103356. Annu Rev Cell Dev Biol. 2003. PMID: 14570581 Review.
Cited by
- Tumor suppressor activity of profilin requires a functional actin binding site.
Wittenmayer N, Jandrig B, Rothkegel M, Schlüter K, Arnold W, Haensch W, Scherneck S, Jockusch BM. Wittenmayer N, et al. Mol Biol Cell. 2004 Apr;15(4):1600-8. doi: 10.1091/mbc.e03-12-0873. Epub 2004 Feb 6. Mol Biol Cell. 2004. PMID: 14767055 Free PMC article. - Profilin II regulates the exocytosis of kainate glutamate receptors.
Mondin M, Carta M, Normand E, Mulle C, Coussen F. Mondin M, et al. J Biol Chem. 2010 Dec 17;285(51):40060-71. doi: 10.1074/jbc.M110.140442. Epub 2010 Oct 11. J Biol Chem. 2010. PMID: 20937818 Free PMC article. - GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition.
Jacob TC, Moss SJ, Jurd R. Jacob TC, et al. Nat Rev Neurosci. 2008 May;9(5):331-43. doi: 10.1038/nrn2370. Nat Rev Neurosci. 2008. PMID: 18382465 Free PMC article. Review. - Gephyrin: a master regulator of neuronal function?
Tyagarajan SK, Fritschy JM. Tyagarajan SK, et al. Nat Rev Neurosci. 2014 Mar;15(3):141-56. doi: 10.1038/nrn3670. Nat Rev Neurosci. 2014. PMID: 24552784 Review. - Molecular architecture of glycinergic synapses.
Dresbach T, Nawrotzki R, Kremer T, Schumacher S, Quinones D, Kluska M, Kuhse J, Kirsch J. Dresbach T, et al. Histochem Cell Biol. 2008 Oct;130(4):617-33. doi: 10.1007/s00418-008-0491-y. Epub 2008 Aug 22. Histochem Cell Biol. 2008. PMID: 18719933 Review.
References
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB ( 2002) Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109 : 509-521. - PubMed
- Bjorkegren C, Rozycki M, Schutt CE, Lindberg U, Karlsson R ( 1993) Mutagenesis of human profilin locates its poly(l-proline)-binding site to a hydrophobic patch of aromatic amino acids. FEBS Lett 333 : 123-126. - PubMed
- Carlsson L, Nystrom L, Sundkvist I, Markey F, Lindberg U ( 1976) Profilin, a low-molecular weight protein controlling actin polymerisability. In: Contractile systems in non-muscle tissues (Perry SV, Margreth A, Adelstein RS, eds), pp 39-49. Amsterdam: North Holland.
- Cramer LP ( 2002) Ena/Vasp: solving a cell motility paradox. Curr Biol 12 : R417-R419. - PubMed
- Di Nardo A, Gareus R, Kwiatkowski D, Witke W ( 2000) Alternative splicing of the mouse profilin II gene generates functionally different profilin isoforms. J Cell Sci 113 : 3795-3803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous