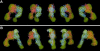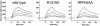Structure of integrin alpha5beta1 in complex with fibronectin - PubMed (original) (raw)
Structure of integrin alpha5beta1 in complex with fibronectin
Junichi Takagi et al. EMBO J. 2003.
Abstract
The membrane-distal headpiece of integrins has evolved to specifically bind large extracellular protein ligands, but the molecular architecture of the resulting complexes has not been determined. We used molecular electron microscopy to determine the three-dimensional structure of the ligand-binding headpiece of integrin alpha5beta1 complexed with fragments of its physiological ligand fibronectin. The density map for the unliganded alpha5beta1 headpiece shows a 'closed' conformation similar to that seen in the alphaVbeta3 crystal structure. By contrast, binding to fibronectin induces an 'open' conformation with a dramatic, approximately 80 degrees change in the angle of the hybrid domain of the beta subunit relative to its I-like domain. The fibronectin fragment binds to the interface between the beta-propeller and I-like domains in the integrin headpiece through the RGD-containing module 10, but direct contact of the synergy-region-containing module 9 to integrin is not evident. This finding is corroborated by kinetic analysis of real-time binding data, which shows that the synergy site greatly enhances k(on) but has little effect on the stability or k(off) of the complex.
Figures
Fig. 1. The truncated α5β1 headpiece retains its functional integrity. (A) The domain organization within the primary structure of integrin α5β1 is shown on the left, and the design of the recombinant soluble α5β1 headpiece is shown on the right. Disulfide-bonded α-helical coiled-coil domains were attached to the C-termini of the truncated subunits to act as a clasp (Takagi et al., 2001). Domains included in the headpiece fragment are color coded as follows: α5 β-propeller repeat domain in pink, α5 thigh domain in yellow, β1 PSI domain in gray, β1 I-like domain in cyan and β1 hybrid domain in red. Domains not resolved in the crystal structure are depicted by dotted lines, and the position of the long-range disulfide bond present in the β subunit is shown below. (B) Binding analysis of Fn9–10 to the α5β1 head fragment by surface plasmon resonance. Clasped (–TEV, dotted lines) or unclasped (+TEV, solid lines) α5β1 headpieces were preincubated with (red lines) or without (black lines) a 3-fold molar excess of TS2/16 Fab fragment for more than 10 min and infused at a concentration of 50 nM onto the sensor surface coated with 650 RU of Fn9–10. Arrows indicate start- and end-points of the injections. (C) Gel filtration chromatography of the α5β1 headpiece with bound ligands. The TEV-cleaved α5β1 headpiece (∼70 pmol) was incubated with 150 pmol of Fn9–10 (blue), Fn7–10 (red) or without ligands (black) in the presence of 1 mM Mn2+ for 1 h and separated on a Superdex 200 column. Chromatograms for 150 pmol Fn7–10 (red dotted) or Fn9–10 (blue dotted) alone are also shown. The elution positions of standard proteins are indicated by arrows (667 kDa, thyroglobulin; 443 kDa, apoferritin; 200 kDa, β-amylase; 67 kDa, serum albumin; 29 kDa, carbonic anhydrase; 12.4 kDa, cytochrome c).
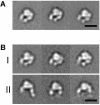
Fig. 2. Projection averages of the α5β1 headpiece obtained by negative stain EM. The unclasped α5β1 headpiece was incubated (A) without or (B) with 1 mM RGD peptide and imaged in the EM. In the +RGD condition, particles with closed (group I) and open (group II) conformations were observed. The three most typical averages for each group are shown, each containing 200–600 particles. Scale bars, 100 Å.
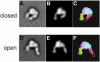
Fig. 3. Two different integrin headpiece conformations. A representative projection average from (A) unliganded and (D) liganded α5β1 headpiece was used to identify the best-correlating projections calculated from a 25 Å density map created from αVβ3 headpiece models (B and E). The model for the open αVβ3 headpiece was prepared as described in the text and Materials and methods section. The models are shown in CPK representation (C and F) using the same color code as in Figure 1A.
Fig. 4. α5β1 head in complex with Fn fragment. Projection averages are shown for (A) Fn7–10 alone, (B) α5β1 complex with Fn7–10 and (C) α5β1 complex with Fn9–10. The projection averages of the α5β1/Fn7–10 complexes are subgrouped (groups I, II and III) based on the orientation of the bound ligand, and several representative raw images are shown below (raw). As in Figure 2, the three most populated averages for each group are shown. The assignment of the FnIII modules (black ovals) in the complex is shown in the schematic drawing to the right. The modules missing in the averages due to linker flexibility are depicted by gray ovals. Scale bars, 100 Å.
Fig. 5. Surface-rendered density maps of the α5β1 headpiece in (A) the unliganded closed and (B) the ligand-bound open conformation. The unmodified headpiece segments of the αVβ3 crystal structure or the open αVβ3 model were manually fitted into the 3D density map of the unliganded and ligand-bound α5β1 headpiece, respectively. Cα worm tracings for αV, β3 and Fn10 segments are colored in red, blue and white, respectively. Views are successive 45° rotations about the vertical figure axis. The figure was generated using DINO (Philippsen, 2002).
Fig. 6. Comparison of the kinetics of α5β1 binding to recombinant Fn7–10 fragment. Either wild-type, R1379A or RPR/AAA synergy mutant Fn7–10 fragments were immobilized on the sensor chip at the same density (500 RU), and full-length α5β1 was injected at 20 µl/min. Traces show increasing concentrations (7.5, 15, 30 and 60 nM) of α5β1 analyte.
Similar articles
- Crystal structure of α5β1 integrin ectodomain: atomic details of the fibronectin receptor.
Nagae M, Re S, Mihara E, Nogi T, Sugita Y, Takagi J. Nagae M, et al. J Cell Biol. 2012 Apr 2;197(1):131-40. doi: 10.1083/jcb.201111077. Epub 2012 Mar 26. J Cell Biol. 2012. PMID: 22451694 Free PMC article. - Structural basis for ligand recognition by RGD (Arg-Gly-Asp)-dependent integrins.
Takagi J. Takagi J. Biochem Soc Trans. 2004 Jun;32(Pt3):403-6. doi: 10.1042/BST0320403. Biochem Soc Trans. 2004. PMID: 15157147 Review. - Metal ion and ligand binding of integrin α5β1.
Xia W, Springer TA. Xia W, et al. Proc Natl Acad Sci U S A. 2014 Dec 16;111(50):17863-8. doi: 10.1073/pnas.1420645111. Epub 2014 Dec 4. Proc Natl Acad Sci U S A. 2014. PMID: 25475857 Free PMC article. - Relating conformation to function in integrin α5β1.
Su Y, Xia W, Li J, Walz T, Humphries MJ, Vestweber D, Cabañas C, Lu C, Springer TA. Su Y, et al. Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):E3872-81. doi: 10.1073/pnas.1605074113. Epub 2016 Jun 17. Proc Natl Acad Sci U S A. 2016. PMID: 27317747 Free PMC article. - Conformational regulation of integrin structure and function.
Shimaoka M, Takagi J, Springer TA. Shimaoka M, et al. Annu Rev Biophys Biomol Struct. 2002;31:485-516. doi: 10.1146/annurev.biophys.31.101101.140922. Epub 2001 Oct 25. Annu Rev Biophys Biomol Struct. 2002. PMID: 11988479 Review.
Cited by
- Structural insights into integrin α5β1 opening by fibronectin ligand.
Schumacher S, Dedden D, Nunez RV, Matoba K, Takagi J, Biertümpfel C, Mizuno N. Schumacher S, et al. Sci Adv. 2021 May 7;7(19):eabe9716. doi: 10.1126/sciadv.abe9716. Print 2021 May. Sci Adv. 2021. PMID: 33962943 Free PMC article. - While the revolution will not be crystallized, biochemistry reigns supreme.
Takizawa Y, Binshtein E, Erwin AL, Pyburn TM, Mittendorf KF, Ohi MD. Takizawa Y, et al. Protein Sci. 2017 Jan;26(1):69-81. doi: 10.1002/pro.3054. Epub 2016 Oct 6. Protein Sci. 2017. PMID: 27673321 Free PMC article. Review. - Cytoskeletal prestress: The cellular hallmark in mechanobiology and mechanomedicine.
Chowdhury F, Huang B, Wang N. Chowdhury F, et al. Cytoskeleton (Hoboken). 2021 Jun;78(6):249-276. doi: 10.1002/cm.21658. Epub 2021 May 1. Cytoskeleton (Hoboken). 2021. PMID: 33754478 Free PMC article. Review. - Crystal structure of α5β1 integrin ectodomain: atomic details of the fibronectin receptor.
Nagae M, Re S, Mihara E, Nogi T, Sugita Y, Takagi J. Nagae M, et al. J Cell Biol. 2012 Apr 2;197(1):131-40. doi: 10.1083/jcb.201111077. Epub 2012 Mar 26. J Cell Biol. 2012. PMID: 22451694 Free PMC article. - Prostacyclin analogs inhibit fibroblast contraction of collagen gels through the cAMP-PKA pathway.
Kamio K, Liu X, Sugiura H, Togo S, Kobayashi T, Kawasaki S, Wang X, Mao L, Ahn Y, Hogaboam C, Toews ML, Rennard SI. Kamio K, et al. Am J Respir Cell Mol Biol. 2007 Jul;37(1):113-20. doi: 10.1165/rcmb.2007-0009OC. Epub 2007 Mar 15. Am J Respir Cell Mol Biol. 2007. PMID: 17363776 Free PMC article.
References
- Altroff H., van Der Walle,C.F., Asselin,J., Fairless,R., Campbell,I.D. and Mardon,H.J. (2001) The eighth FIII domain of human fibronectin promotes integrin α5β1 binding via stabilization of the ninth FIII domain. J. Biol. Chem., 276, 38885–38892. - PubMed
- Aota S., Nomizu,M. and Yamada,K.M. (1994) The short amino acid sequence Pro–His–Ser–Arg–Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem., 269, 24756–24761. - PubMed
- Baron M., Main,A.L., Driscoll,P.C., Mardon,H.J., Boyd,J. and Campbell,I.D. (1992) 1H NMR assignment and secondary structure of the cell adhesion type III module of fibronectin. Biochemistry, 31, 2068–2073. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials