New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products - PubMed (original) (raw)
New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products
David A Kocisko et al. J Virol. 2003 Oct.
Abstract
Transmissible spongiform encephalopathies (TSEs) are fatal, untreatable neurodegenerative diseases associated with the accumulation of a disease-specific form of prion protein (PrP) in the brain. One approach to TSE therapeutics is the inhibition of PrP accumulation. Indeed, many inhibitors of the accumulation of PrP associated with scrapie (PrP(Sc)) in scrapie-infected mouse neuroblastoma cells (ScN(2)a) also have antiscrapie activity in rodents. To expedite the search for potential TSE therapeutic agents, we have developed a high-throughput screening assay for PrP(Sc) inhibitors using ScN(2)a cells in a 96-well format. A library of 2000 drugs and natural products was screened in ScN(2)a cells infected with scrapie strain RML (Chandler) or 22L. Forty compounds were found to have concentrations causing 50% inhibition (IC(50)s) of PrP(Sc) accumulation of <or=10 microM against both strains. Seventeen had IC(50)s of <or=1 microM against both strains. Several classes of compounds were represented in the 17 most potent inhibitors, including naturally occurring polyphenols (e.g., tannic acid and tea extracts), phenothiazines, antihistamines, statins, and antimalarial compounds. These 17 compounds were also evaluated in a solid-phase cell-free hamster PrP conversion assay. Only the polyphenols inhibited the cell-free reaction, and their IC(50)s were near 100 nM. Several of the new PrP(Sc) inhibitors cross the blood-brain barrier and thus have potential to be effective after TSE infection reaches the brain. The fact that many are either approved human drugs or edible natural products should facilitate their use in animal testing and clinical trials.
Figures
FIG. 1.
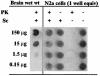
Dot blot of brain-derived PrPSc and ScN2a cell-derived PrPC and PrPSc. The wells shown are from a single membrane visualized with primary antibody 6H4. The samples in the first lane contain the indicated brain wet-weight (wet wt) equivalents in a lysate from a hamster clinically ill from infection with scrapie strain 263K. The second and third lanes from the left contain lysates from RML-infected ScN2a cells (one well equivalent). The fourth and fifth lanes contain lysates from uninfected N2a cells. PrPC from uninfected cells was detected without any PK treatment.
FIG. 2.
Partial 96-well dot blot showing the PK-resistant PrP signal visualized with primary antibody 6B10. Signals from untreated control (Cont) cells and curcumin-inhibited (Cur) cells are indicated. The latter were incubated in the presence of 10 μM curcumin, a known inhibitor of PrPSc in RML-infected cells (6). Other dots represent signals from ScN2a cells after incubation with 10 μM concentrations of various compounds. Some of these spots have an intensity comparable to that of controls, indicating no inhibition of PrPSc formation. Others that are less intense were due to compounds with various inhibitory strengths or toxicities.
FIG. 3.
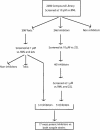
Flowchart of the screening of The Spectrum Collection compound library.
FIG. 4.
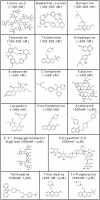
Structures of compounds in The Spectrum Collection with IC50s of >1 and ≤10 μM against both the RML and 22L scrapie strains, listed in approximate alphabetical order. 2,3,5,7,3′,4′-penta-, 2,3,5,7,3′,4′-pentahydroxyflavan.
FIG. 5.
Structures of compounds in The Spectrum Collection with IC50s of ≤1 μM against both the RML and 22L strains of scrapie. Compounds are arranged from low to high approximate IC50s.
FIG. 6.
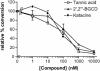
Inhibition of solid-phase cell-free PrP conversion by polyphenols. The conversion relative to that in control reactions is plotted against the concentration of polyphenol added to the reaction. 2′,2′"-BGCD, 2′,2′"-bisepigallocatechin digallate.
FIG. 7.
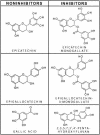
Structural comparisons of inhibitory and noninhibitory polyphenols. Epicatechin and epigallocatechin were not inhibitors until the addition of a gallate, which was not an inhibitor on its own. Compared to epicatechin, the inhibitor 2,3,5,7,3′,4′-pentahydroxyflavan has one additional conjugated double bond and an additional hydroxyl group. The double-ring system in the flavan should be more planar than the corresponding rings in epicatechin.
References
- Beranger, F., A. Mange, J. Solassol, and S. Lehmann. 2001. Cell culture models of transmissible spongiform encephalopathies. Biochem. Biophys. Res. Commun. 289**:**311-316. -PubMed
- Caughey, B., and B. Chesebro. 2001. Transmissible spongiform encephalopathies and prion protein interconversions. Adv. Virus Res. 56**:**277-311. -PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials