Significance in replication of the terminal nucleotides of the flavivirus genome - PubMed (original) (raw)
Significance in replication of the terminal nucleotides of the flavivirus genome
Alexander A Khromykh et al. J Virol. 2003 Oct.
Abstract
Point mutations that resulted in a substitution of the conserved 3'-penultimate cytidine in genomic RNA or the RNA negative strand of the self-amplifying replicon of the Flavivirus Kunjin virus completely blocked in vivo replication. Similarly, substitutions of the conserved 3'-terminal uridine in the RNA negative or positive strand completely blocked replication or caused much-reduced replication, respectively. The same preference for cytidine in the 3'-terminal dinucleotide was noted in reports of the in vitro activity of the RNA-dependent RNA polymerase (RdRp) for the other genera of Flaviviridae that also employ a double-stranded RNA (dsRNA) template to initiate asymmetric semiconservative RNA positive-strand synthesis. The Kunjin virus replicon results were interpreted in the context of a proposed model for initiation of RNA synthesis based on the solved crystal structure of the RdRp of phi6 bacteriophage, which also replicates efficiently using a dsRNA template with conserved 3'-penultimate cytidines and a 3'-terminal pyrimidine. A previously untested substitution of the conserved pentanucleotide at the top of the 3'-terminal stem-loop of all Flavivirus species also blocked detectable in vivo replication of the Kunjin virus replicon RNA.
Figures
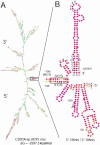
FIG.1.
Computer-predicted model showing the topology of the RNA of the KUN replicon C20DXrep, prepared by using the mfold program and presented as the global MinE structure with bases colored according to their P-num values (38). (A) Model of the complete genome. (B) Enlargement of the inset region in panel A that represents only the 5′-terminal 146 nucleotides (nts) (numbering from 5′ is 1 to 146) and the 3′-terminal 106 nucleotides (numbering −1 to −106 from the terminus). The stem-loop structures in the enlarged inset region are slightly rearranged so the structures of the 5′- and 3′-terminal regions are more clearly depicted. CS identifies the base-paired cyclization sequence (see text). Bases indicated as A, C, G, or U are shown for the terminal nucleotides, the AUG translation initiation codon (at positions 97 to 99), the base-paired region in the lower portion of the 3′-terminal stem-loop, the _Flavivirus_-conserved pentanucleotide at the top of this loop, and the proposed pseudoknot sequences. Low P-num values (<3%) are represented by red bases, while increasing P-num values are represented by the colors orange, yellow, green (medium), and blue through black (high).
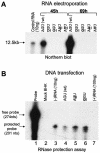
FIG. 2.
Analysis of RNA copied from 3′-mutated KUN replicon RNA by Northern blotting. Approximately 10 μg of total cell RNA was extracted from BHK cells at 45 and 69 h after transfection with 5 μg of KUN replicon RNAs mutated in the penultimate cytidine or the 3′-terminal uridine and subjected to Northern blot hybridization with 32P-labeled cDNA probes detecting replicon RNA and β-actin mRNA as previously described (31). The mutated nucleotides are shown in boldface and underlined. Densitometry analysis of the blots is shown in Table 1.
FIG. 3.
Analysis of RNA copied from 5′-mutated replicon RNA. (A) Northern blot analysis with KUN replicon-specific 32P-labeled cDNA probe of ∼10 μg of total cell RNA extracted from BHK cells at 45 and 69 h after transfection with replicon RNAs containing native (wild type [wt]) and mutated 5′-terminal ribonucleotides. (B) Detection of KUN replicon RNA negative-strand synthesis in BHK cells at 48 h after transfection with plasmid DNAs containing KUN replicon cDNA with native and mutated 5′-terminal nucleotides. The probe was a 32P-labeled 274-nucleotide (nts) positive-sense RNA from the NS4A region transcribed in vitro, and the RNase protection assay was performed with an RPA-III kit, essentially as described by the manufacturer (Ambion, Inc., Austin, Tex.).
Similar articles
- Kunjin RNA replication and applications of Kunjin replicons.
Westaway EG, Mackenzie JM, Khromykh AA. Westaway EG, et al. Adv Virus Res. 2003;59:99-140. doi: 10.1016/s0065-3527(03)59004-2. Adv Virus Res. 2003. PMID: 14696328 Review. - Structure and function of the 3' terminal six nucleotides of the west nile virus genome in viral replication.
Tilgner M, Shi PY. Tilgner M, et al. J Virol. 2004 Aug;78(15):8159-71. doi: 10.1128/JVI.78.15.8159-8171.2004. J Virol. 2004. PMID: 15254187 Free PMC article. - Dual Role of a Viral Polymerase in Viral Genome Replication and Particle Self-Assembly.
Sun X, Ilca SL, Huiskonen JT, Poranen MM. Sun X, et al. mBio. 2018 Oct 2;9(5):e01242-18. doi: 10.1128/mBio.01242-18. mBio. 2018. PMID: 30279282 Free PMC article. - [Kunjin virus replicon--a novel viral vector].
Li S, Li X, Qin E, Qin C. Li S, et al. Sheng Wu Gong Cheng Xue Bao. 2011 Feb;27(2):141-6. Sheng Wu Gong Cheng Xue Bao. 2011. PMID: 21650037 Review. Chinese.
Cited by
- Structural and functional studies of the promoter element for dengue virus RNA replication.
Lodeiro MF, Filomatori CV, Gamarnik AV. Lodeiro MF, et al. J Virol. 2009 Jan;83(2):993-1008. doi: 10.1128/JVI.01647-08. Epub 2008 Nov 12. J Virol. 2009. PMID: 19004935 Free PMC article. - Molecular Insights into the Flavivirus Replication Complex.
van den Elsen K, Quek JP, Luo D. van den Elsen K, et al. Viruses. 2021 May 21;13(6):956. doi: 10.3390/v13060956. Viruses. 2021. PMID: 34064113 Free PMC article. Review. - Feline calicivirus VP2 is essential for the production of infectious virions.
Sosnovtsev SV, Belliot G, Chang KO, Onwudiwe O, Green KY. Sosnovtsev SV, et al. J Virol. 2005 Apr;79(7):4012-24. doi: 10.1128/JVI.79.7.4012-4024.2005. J Virol. 2005. PMID: 15767403 Free PMC article. - Repression and derepression of minus-strand synthesis in a plus-strand RNA virus replicon.
Zhang G, Zhang J, Simon AE. Zhang G, et al. J Virol. 2004 Jul;78(14):7619-33. doi: 10.1128/JVI.78.14.7619-7633.2004. J Virol. 2004. PMID: 15220437 Free PMC article. - The topology of bulges in the long stem of the flavivirus 3' stem-loop is a major determinant of RNA replication competence.
Yu L, Markoff L. Yu L, et al. J Virol. 2005 Feb;79(4):2309-24. doi: 10.1128/JVI.79.4.2309-2324.2005. J Virol. 2005. PMID: 15681432 Free PMC article.
References
- Bartholomeusz, A. I., and P. J. Wright. 1993. Synthesis of dengue virus RNA in vitro: initiation and the involvement of proteins NS3 and NS5. Arch. Virol. 128:111-121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials