Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species - PubMed (original) (raw)
Review
. 2003 Nov 25;100 Suppl 2(Suppl 2):14555-61.
doi: 10.1073/pnas.1934677100. Epub 2003 Sep 11.
Affiliations
- PMID: 12970466
- PMCID: PMC304118
- DOI: 10.1073/pnas.1934677100
Review
Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species
Gregory L Challis et al. Proc Natl Acad Sci U S A. 2003.
Abstract
In this article we briefly review theories about the ecological roles of microbial secondary metabolites and discuss the prevalence of multiple secondary metabolite production by strains of Streptomyces, highlighting results from analysis of the recently sequenced Streptomyces coelicolor and Streptomyces avermitilis genomes. We address this question: Why is multiple secondary metabolite production in Streptomyces species so commonplace? We argue that synergy or contingency in the action of individual metabolites against biological competitors may, in some cases, be a powerful driving force for the evolution of multiple secondary metabolite production. This argument is illustrated with examples of the coproduction of synergistically acting antibiotics and contingently acting siderophores: two well-known classes of secondary metabolite. We focus, in particular, on the coproduction of beta-lactam antibiotics and beta-lactamase inhibitors, the coproduction of type A and type B streptogramins, and the coregulated production and independent uptake of structurally distinct siderophores by species of Streptomyces. Possible mechanisms for the evolution of multiple synergistic and contingent metabolite production in Streptomyces species are discussed. It is concluded that the production by Streptomyces species of two or more secondary metabolites that act synergistically or contingently against biological competitors may be far more common than has previously been recognized, and that synergy and contingency may be common driving forces for the evolution of multiple secondary metabolite production by these sessile saprophytes.
Figures
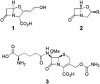
Fig. 1.
Structures of clavulanic acid (1), clavams (R = variable group) (2), and cephamycin C (3), coproduced by several Streptomyces species. 1 and 3 act synergistically to inhibit cell wall biosynthesis in β-lactam-resistant bacteria.
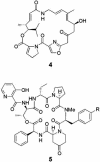
Fig. 2.
Structures of pristinamycin I (4) and II (R = variable group) (5) components, which inhibit protein synthesis in bacteria by binding synergistically to the ribosome.
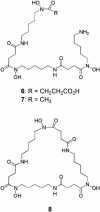
Fig. 3.
Structures of desferrioxamine siderophores typically produced by Streptomyces species.
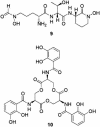
Fig. 4.
Predicted structure of coelichelin (9) and structure of enterobactin (10), siderophores of diverse structure coproduced with desferrioxamines by some Streptomyces species that are thought to provide a contingency plan for iron uptake in the event of desferrioxamine piracy.
Similar articles
- Competition Sensing Changes Antibiotic Production in Streptomyces.
Westhoff S, Kloosterman AM, van Hoesel SFA, van Wezel GP, Rozen DE. Westhoff S, et al. mBio. 2021 Feb 9;12(1):e02729-20. doi: 10.1128/mBio.02729-20. mBio. 2021. PMID: 33563841 Free PMC article. - Multiple biosynthetic and uptake systems mediate siderophore-dependent iron acquisition in Streptomyces coelicolor A3(2) and Streptomyces ambofaciens ATCC 23877.
Barona-Gómez F, Lautru S, Francou FX, Leblond P, Pernodet JL, Challis GL. Barona-Gómez F, et al. Microbiology (Reading). 2006 Nov;152(Pt 11):3355-3366. doi: 10.1099/mic.0.29161-0. Microbiology (Reading). 2006. PMID: 17074905 - Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites.
Omura S, Ikeda H, Ishikawa J, Hanamoto A, Takahashi C, Shinose M, Takahashi Y, Horikawa H, Nakazawa H, Osonoe T, Kikuchi H, Shiba T, Sakaki Y, Hattori M. Omura S, et al. Proc Natl Acad Sci U S A. 2001 Oct 9;98(21):12215-20. doi: 10.1073/pnas.211433198. Epub 2001 Sep 25. Proc Natl Acad Sci U S A. 2001. PMID: 11572948 Free PMC article. - Genome mining of Streptomyces ambofaciens.
Aigle B, Lautru S, Spiteller D, Dickschat JS, Challis GL, Leblond P, Pernodet JL. Aigle B, et al. J Ind Microbiol Biotechnol. 2014 Feb;41(2):251-63. doi: 10.1007/s10295-013-1379-y. Epub 2013 Nov 21. J Ind Microbiol Biotechnol. 2014. PMID: 24258629 Review. - Challenges in the Heterologous Production of Antibiotics in Streptomyces.
Bekiesch P, Basitta P, Apel AK. Bekiesch P, et al. Arch Pharm (Weinheim). 2016 Aug;349(8):594-601. doi: 10.1002/ardp.201600058. Epub 2016 Jun 3. Arch Pharm (Weinheim). 2016. PMID: 27258165 Review.
Cited by
- Triggers and cues that activate antibiotic production by actinomycetes.
Zhu H, Sandiford SK, van Wezel GP. Zhu H, et al. J Ind Microbiol Biotechnol. 2014 Feb;41(2):371-86. doi: 10.1007/s10295-013-1309-z. Epub 2013 Aug 2. J Ind Microbiol Biotechnol. 2014. PMID: 23907251 Review. - Comparative Genomics and Metabolomics Analyses of Clavulanic Acid-Producing Streptomyces Species Provides Insight Into Specialized Metabolism.
AbuSara NF, Piercey BM, Moore MA, Shaikh AA, Nothias LF, Srivastava SK, Cruz-Morales P, Dorrestein PC, Barona-Gómez F, Tahlan K. AbuSara NF, et al. Front Microbiol. 2019 Nov 8;10:2550. doi: 10.3389/fmicb.2019.02550. eCollection 2019. Front Microbiol. 2019. PMID: 31787949 Free PMC article. - Genomic Insights Into Plant-Growth-Promoting Potentialities of the Genus Frankia.
Nouioui I, Cortés-Albayay C, Carro L, Castro JF, Gtari M, Ghodhbane-Gtari F, Klenk HP, Tisa LS, Sangal V, Goodfellow M. Nouioui I, et al. Front Microbiol. 2019 Jul 4;10:1457. doi: 10.3389/fmicb.2019.01457. eCollection 2019. Front Microbiol. 2019. PMID: 31333602 Free PMC article. - Type I polyketide synthases may have evolved through horizontal gene transfer.
Ginolhac A, Jarrin C, Robe P, Perrière G, Vogel TM, Simonet P, Nalin R. Ginolhac A, et al. J Mol Evol. 2005 Jun;60(6):716-25. doi: 10.1007/s00239-004-0161-1. Epub 2005 May 16. J Mol Evol. 2005. PMID: 15909225 - Catalytic promiscuity in the biosynthesis of cyclic peptide secondary metabolites in planktonic marine cyanobacteria.
Li B, Sher D, Kelly L, Shi Y, Huang K, Knerr PJ, Joewono I, Rusch D, Chisholm SW, van der Donk WA. Li B, et al. Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10430-5. doi: 10.1073/pnas.0913677107. Epub 2010 May 17. Proc Natl Acad Sci U S A. 2010. PMID: 20479271 Free PMC article.
References
- Demain, A. & Fang, A. (2000) in History of Modern Biotechnology, ed. Fichter, A. (Springer, Berlin), Vol. 1, pp. 2-39.
- Berdy, J. (1995) in Proceedings of the Ninth International Symposium on the Biology of the Actinomycetes, eds. Debabov, V. G., Dudnik, Y. V. & Danilenko, V. N. (All-Russia Research Institute for Genetics and Selection of Industrial Microrganisms, Moscow), pp. 13-34.
- Williams, D. H., Stone, M. J., Hauck, P. R. & Rahman, S. K. (1989) J. Nat. Products 52, 1189-1208. - PubMed
- Donadio, S., Staver, M. J., McAlpine, J. B., Swanson, S. J. & Katz, L. (1991) Proc. Natl. Acad. Sci. USA 252, 675-679. - PubMed
- Firn, R. D. & Jones, C. G. (2000) Mol. Microbiol. 37, 989-994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources