The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility - PubMed (original) (raw)
. 2003 Sep 15;162(6):1111-22.
doi: 10.1083/jcb.200212157.
Stefan Liebner, Radiosa Gallini, Adriana Zanetti, Giovanna Balconi, Alessandro Corsi, Paolo Bianco, Hartwig Wolburg, Robert Moore, Boussadia Oreda, Rolf Kemler, Elisabetta Dejana
Affiliations
- PMID: 12975353
- PMCID: PMC2172846
- DOI: 10.1083/jcb.200212157
The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility
Anna Cattelino et al. J Cell Biol. 2003.
Abstract
Using the Cre/loxP system we conditionally inactivated beta-catenin in endothelial cells. We found that early phases of vasculogenesis and angiogenesis were not affected in mutant embryos; however, vascular patterning in the head, vitelline, umbilical vessels, and the placenta was altered. In addition, in many regions, the vascular lumen was irregular with the formation of lacunae at bifurcations, vessels were frequently hemorrhagic, and fluid extravasation in the pericardial cavity was observed. Cultured beta-catenin -/- endothelial cells showed a different organization of intercellular junctions with a decrease in alpha-catenin in favor of desmoplakin and marked changes in actin cytoskeleton. These changes paralleled a decrease in cell-cell adhesion strength and an increase in paracellular permeability. We conclude that in vivo, the absence of beta-catenin significantly reduces the capacity of endothelial cells to maintain intercellular contacts. This may become more marked when the vessels are exposed to high or turbulent flow, such as at bifurcations or in the beating heart, leading to fluid leakage or hemorrhages.
Figures
Figure 1.
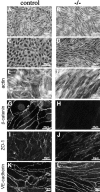
Cre recombinase efficiently recombines the β -catenin flox allele in vivo. (A) Genomic PCR on E10.5 embryos from Tie2-Cre x β-catenin flox/flox crossings for the _β_-catenin gene and for Tie2-Cre gene. The four lanes represent the four different genotypes obtained: β-catenin flox del/flox, Cre − (+/−, lane 1), β-catenin flox del/flox, Cre + (−/−, lane 2), β-catenin flox/wt, Cre − (+/+, lane 3), and β-catenin flox/wt, Cre + (+/−, lane 4). In this last lane, note the presence of the recombined β-catenin flox del band when the flox allele is in the presence of Tie2-Cre recombinase. (B) Western blot of β-catenin protein in endothelial cell lines established from wt (control) and β-catenin flox/flox del, Tie2-Cre + embryos (−/−). (C-J) Immunofluorescence for anti-PECAM and anti-β-catenin antibodies on serial cryosections from wild-type (+/+, control) and β-catenin flox/flox del, Tie2-Cre + (−/−, mutant) E10.5 embryos. Heart sections show positive staining of the endocardium (e) with PECAM antibodies in both control (C) and β-catenin −/− (E) animals. β-Catenin staining is undetectable in mutant embryos (F). β-Catenin is also absent in the dorsal aorta (G and H, Ao) and in the yolk sac vessels (I and J, arrow) of the mutant embryos. Bar in H refers to C–H, and bar in J refers to I and J.
Figure 2.
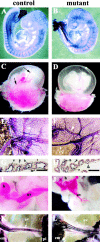
Phenotypic defects in endothelial β _-catenin_–null embryos. (A and B) E9.5 embryos after whole-mount immunohistochemistry with anti-PECAM antibodies. No major differences can be observed in the vascular phenotype of control (A) and mutant (B) embryos at this stage. (C–J) E10.5 embryos. Although major vitelline vessels are visible in control embryos after dissection (C, arrows), the mutant yolk sac appeares paler (D). In E–H, K, and L, whole-mount staining for PECAM was performed. In E (control) and F (mutant), note the difference in diameter of vitelline (vv) and major vessels (arrowheads) in the yolk sac. In both control (G) and mutant embryos (H), PECAM and nuclear fast red staining of yolk sac sections shows essentially indistinguishable vessels with the presence of endothelial (e), mesothelial (m), blood (b), and endodermal cells (n). (I–L) Umbilical vessels (uv) in 10.5 (I and J) and 11.5 embryos (K and L). In the mutants, umbilical vessels are smaller in diameter, ramified, or anastomosed (J and L) as compared with the control (I and K). h, heart; em, embryo; pl, placenta. Bars, 100 μm.
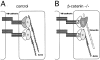
Figure 3.
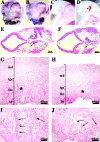
Vascular defects in the placenta and head of endothelial β _-catenin_–null embryos. Whole-mount immunohistochemistry for anti-PECAM antibody in E10.5 embryos (A and B). In the head, mutant embryos (B and D) compared with controls (A and C) show a less organized vascular network with vessels of irregular diameter and shape. In mutants, blind ending vessels (B, arrowhead) and lacunae-like bifurcations (D, arrowhead) can be often observed. PECAM and nuclear fast red staining in two serial transverse sections, separated by 60 μm, of E10.5 mutant head (E and F). The arrows point to the diameter of the internal carotid artery, which is significantly different in the two sections. Placenta histology of control (G and I) and mutant E10.5 embryos (H and J) shows that, although the maternal decidua (md), the chorionic plate (cp), and the trophoblast giant cells (tgc) are comparable, the labyrinthine layer (lbr) is reduced in mutants. I and J represent higher magnifications of the zones indicated by the asterisks in G and H, respectively. Embryonic blood vessels (arrows), are substantially more abundant in the labyrinthine zone of the control (I), than in the mutant (J).
Figure 4.
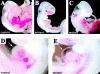
Hemorrhages and fluid extravasation in β -catenin mutant embryos. E10.5 control (A and D) and mutant embryos (B, C, and E). Arrowheads in B and C indicate regions of extended hemorrhages. In the mutants, we observed fluid accumulation in the pericardial cavity (arrow in E in comparison to controls in D) suggesting increase of permeability of cardiac microvasculature.
Figure 5.
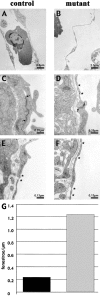
Thinner endocardium and increased number of fenestrations in β _-catenin_–null embryos. In electron micrographs, the endocardial lining of the heart appear continuously thinner in the null embryos compared with control littermates (compare A with B), whereas the myocardium is equally organized (not depicted). Ultrathin sections of yolk sac vessels of control and mutant embryos show similar features as the heart endocardium, with thinner endothelial walls and less organized junctions in _β-catenin_–deficient embryos. In the latter, the inter-endothelial junctions show a reduced junctional overlapping (see arrows), as compared with controls (C and D; asterisks point to fenestrations). Furthermore, a higher incidence of fenestrations (asterisks) in the yolk sac vasculature of null embryos can be observed (compare D, E, and F), which are quantified as number of fenestrae per length unit of endothelial wall (G; 10 pictures counted for each genotype).
Figure 6.
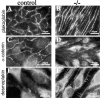
Morphological differences and junctional organization in control and β -catenin −/− endothelial cells in culture. A represents β-catenin flox/flox del (control) endothelial cells, whereas B represents the same cells after infection with an adeno vector expressing Cre recombinase (β-catenin flox del/flox del, or −/−). In C, E, and G, endothelial cells cultured from control embryos are shown, whereas D, F, and H show endothelial cells from β-catenin −/− embryos. Although control endothelial cells exhibit classical cobblestone morphology (A and C), β-catenin −/− cells are more elongated and have a fibroblastoid/mesenchymal morphology (B and D). Phalloidin staining shows more elongated and thinner bundles of actin filaments in β-catenin −/− cells (F), as compared with controls (E). β-Catenin staining is undetectable at intercellular contacts of −/− cells (compare G and H), whereas ZO-1 (I and J) and VE-cadherin (K and L) are correctly organized at intercellular junctions.
Figure 7.
Immunostaining of junctional proteins in β-catenin −**/**− endothelial cells. Although plakoglobin is correctly organized at cell–cell contacts in β-catenin −/− embryos (compare A and B), α-catenin antibodies give a very faint and discontinuous staining at cell–cell contacts in β-catenin −/−, as compared with control cells (compare C and D). Note the presence of desmoplakin at intracellular junctions only in β-catenin −/− cells (F, arrows), whereas in control cells, the same antibody gives only a faint and diffuse staining (E).
Figure 8.
Biochemical characterization of the junctional complex. (A) Association of VE-cadherin with α, β-catenins, and plakoglobin. Endothelial cell extracts from control and β-catenin −/− cells were immunoprecipitated with antibodies to VE-cadherin. The immunoprecipitates (IP) and an equal amount of total protein extracts (Total) from the two cell types were immunoblotted (IB) with the antibodies indicated on the left. Note the decrease in the level of both total and VE-cadherin-bound α-catenin. (B) Increase in desmoplakins level in β-catenin −/− and in control cell lysates. Arrowheads indicate the bands corresponding to desmoplakin I (250 kD) and II (220 kD).
Figure 9.
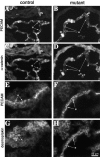
Junctional organization in control and mutant embryos. Cryosections from E10.5 embryos were analyzed for the codistribution of PECAM (A and B) and α-catenin (C and D). PECAM is equally expressed in control (A) and mutant embryos (B), and in some regions it is possible to evidentiate its localization at cell–cell contacts (arrows). In contrast, in mutant embryos α-catenin shows a weaker endothelial staining and colocalization with PECAM at intercellular junctions is rare (compare C and D, arrows). Desmoplakin is detectable in endothelial cells in _β-catenin_–null embryos and colocalizes with PECAM at cell–cell contacts (F and H). In control embryos desmoplakin staining was negative in endothelial cells (E and G). e, endocardium. Bar in H refers to A–H.
Figure 10.
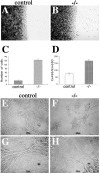
Cell–cell adhesion strength in control and mutant endothelial cells. (A–C) Analysis of endothelial cell migration into a wound. Crystal violet staining of control (A) and β-catenin −/− (B) endothelial cells 8 h after mechanical wounding of the monolayer. C reports the number of migrating single cells into the wound at 8 h. (D) Permeability across endothelial cell monolayers. β-Catenin −/− confluent endothelial cells show a higher passage of FITC-dextran as compared with the control cells. In C and D, columns represent means ± SEM of quadruplicates of a typical experiments out of three performed. (E–H) _β-catenin_–null endothelial cells (−/−) form only small, disorganized aggregates interconnected with thin, single-cell elongations on Matrigel™ matrix (G and H), whereas control endothelial cells are able to organize into aggregates and bundles and show a typical cordlike structure on Matrigel™ matrix (E and F).
Figure 11.
Proposed model for the molecular reorganization of endothelial intercellular junctions in the absence of β-catenin. (A) In the presence of β-catenin (β), α-catenin (α) is normally expressed at cell–cell junctions and connects VE-cadherin/β-catenin or VE-cadherin/plakoglobin (PG) complex to actin cytoskeleton. (B) In the absence of β-catenin, the decrease in α-catenin, accompanied by increase in desmoplakin (DP), may lead to a shift from α-catenin and actin based junctions to desmoplakin and vimentin-based complexus adhaerentes.
Similar articles
- The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin).
Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. Lampugnani MG, et al. J Cell Biol. 1995 Apr;129(1):203-17. doi: 10.1083/jcb.129.1.203. J Cell Biol. 1995. PMID: 7698986 Free PMC article. - Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-to-cell adherens junctions.
Del Maschio A, Zanetti A, Corada M, Rival Y, Ruco L, Lampugnani MG, Dejana E. Del Maschio A, et al. J Cell Biol. 1996 Oct;135(2):497-510. doi: 10.1083/jcb.135.2.497. J Cell Biol. 1996. PMID: 8896605 Free PMC article. - VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: a pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions.
Kowalczyk AP, Navarro P, Dejana E, Bornslaeger EA, Green KJ, Kopp DS, Borgwardt JE. Kowalczyk AP, et al. J Cell Sci. 1998 Oct;111 ( Pt 20):3045-57. doi: 10.1242/jcs.111.20.3045. J Cell Sci. 1998. PMID: 9739078 - Endothelial adhesion molecules in the development of the vascular tree: the garden of forking paths.
Bazzoni G, Dejana E, Lampugnani MG. Bazzoni G, et al. Curr Opin Cell Biol. 1999 Oct;11(5):573-81. doi: 10.1016/s0955-0674(99)00023-x. Curr Opin Cell Biol. 1999. PMID: 10508655 Review.
Cited by
- Transgenic expression of FoxM1 promotes endothelial repair following lung injury induced by polymicrobial sepsis in mice.
Huang X, Zhao YY. Huang X, et al. PLoS One. 2012;7(11):e50094. doi: 10.1371/journal.pone.0050094. Epub 2012 Nov 20. PLoS One. 2012. PMID: 23185540 Free PMC article. - PECAM-1 affects GSK-3beta-mediated beta-catenin phosphorylation and degradation.
Biswas P, Canosa S, Schoenfeld D, Schoenfeld J, Li P, Cheas LC, Zhang J, Cordova A, Sumpio B, Madri JA. Biswas P, et al. Am J Pathol. 2006 Jul;169(1):314-24. doi: 10.2353/ajpath.2006.051112. Am J Pathol. 2006. PMID: 16816383 Free PMC article. - Regulation of endothelial cell cytoskeletal reorganization by a secreted frizzled-related protein-1 and frizzled 4- and frizzled 7-dependent pathway: role in neovessel formation.
Dufourcq P, Leroux L, Ezan J, Descamps B, Lamazière JM, Costet P, Basoni C, Moreau C, Deutsch U, Couffinhal T, Duplàa C. Dufourcq P, et al. Am J Pathol. 2008 Jan;172(1):37-49. doi: 10.2353/ajpath.2008.070130. Epub 2007 Dec 21. Am J Pathol. 2008. PMID: 18156211 Free PMC article. - Stable vascular connections and remodeling require full expression of VE-cadherin in zebrafish embryos.
Montero-Balaguer M, Swirsding K, Orsenigo F, Cotelli F, Mione M, Dejana E. Montero-Balaguer M, et al. PLoS One. 2009 Jun 3;4(6):e5772. doi: 10.1371/journal.pone.0005772. PLoS One. 2009. PMID: 19503615 Free PMC article. - Regulatory pathways affecting vascular stabilization via VE-cadherin dynamics: insights from zebrafish (Danio rerio).
Eisa-Beygi S, Macdonald RL, Wen XY. Eisa-Beygi S, et al. J Cereb Blood Flow Metab. 2014 Sep;34(9):1430-3. doi: 10.1038/jcbfm.2014.128. Epub 2014 Jul 16. J Cereb Blood Flow Metab. 2014. PMID: 25027310 Free PMC article. Review.
References
- Aberle, H., H. Schwartz, and R. Kemler. 1996. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J. Cell. Biochem. 61:514–523. - PubMed
- Balconi, G., R. Spagnuolo, and E. Dejana. 2000. Development of endothelial cell lines from embry Beck onic stem cells: A tool for studying genetically manipulated endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 20:1443–1451. - PubMed
- Behrens, J., J.P. von Kries, M. Kühl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 382:638–642. - PubMed
- Brault, V., R. Moore, S. Kutsch, M. Ishibashi, D.H. Rowitch, A.P. McMahon, L. Sommer, O. Boussadia, and R. Kemler. 2001. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 128:1253–1264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases