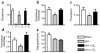A crucial role for thiol antioxidants in estrogen-deficiency bone loss - PubMed (original) (raw)
A crucial role for thiol antioxidants in estrogen-deficiency bone loss
Jenny M Lean et al. J Clin Invest. 2003 Sep.
Abstract
The mechanisms through which estrogen prevents bone loss are uncertain. Elsewhere, estrogen exerts beneficial actions by suppression of reactive oxygen species (ROS). ROS stimulate osteoclasts, the cells that resorb bone. Thus, estrogen might prevent bone loss by enhancing oxidant defenses in bone. We found that glutathione and thioredoxin, the major thiol antioxidants, and glutathione and thioredoxin reductases, the enzymes responsible for maintaining them in a reduced state, fell substantially in rodent bone marrow after ovariectomy and were rapidly normalized by exogenous 17-beta estradiol. Moreover, administration of N-acetyl cysteine (NAC) or ascorbate, antioxidants that increase tissue glutathione levels, abolished ovariectomy-induced bone loss, while l-buthionine-(S,R)-sulphoximine (BSO), a specific inhibitor of glutathione synthesis, caused substantial bone loss. The 17-beta estradiol increased glutathione and glutathione and thioredoxin reductases in osteoclast-like cells in vitro. Furthermore, in vitro NAC prevented osteoclast formation and NF-kappaB activation. BSO and hydrogen peroxide did the opposite. Expression of TNF-alpha, a target for NF-kappaB and a cytokine strongly implicated in estrogen-deficiency bone loss, was suppressed in osteoclasts by 17-beta estradiol and NAC. These observations strongly suggest that estrogen deficiency causes bone loss by lowering thiol antioxidants in osteoclasts. This directly sensitizes osteoclasts to osteoclastogenic signals and entrains ROS-enhanced expression of cytokines that promote osteoclastic bone resorption.
Figures
Figure 1
Estrogen maintains thiol antioxidant system in rat bone marrow. (a and b) Ovariectomy (ovx) decreased while 17-β estradiol (βE2) (10 μg/kg) restored glutathione (nmol/mg protein) and glutathione reductase (mU/mg protein) in bone marrow (r, reduced glutathione; o, oxidized glutathione). (c and d) Ovariectomy suppressed thioredoxin (nmol/mg protein) and thioredoxin reductase (mU/mg protein) in bone marrow, while 17-β estradiol normalized both. (e) 17-α estradiol (αE2) was without significant effect on glutathione levels. Thiol levels and enzymes were also measured in liver and spleen and did not differ significantly between groups. *P < 0.05 versus all other groups. Data expressed as mean ± SEM; n = 6 per group. These experiments were repeated twice in mice and similarly showed significant changes in the levels of antioxidant defense components in bone marrow after ovariectomy, which were normalized by 17-β estradiol.
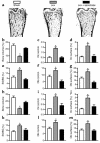
Figure 2
Ascorbate and NAC prevent bone loss in ovariectomized mice. (a–g) Ascorbate (2 mmol/kg/day) prevents ovariectomy-induced bone loss in mice. (a) Representative images of microscope sections of femora from mice subjected to sham ovariectomy (sham) or ovariectomy (ovx) and treated for 2 weeks with ascorbate (1 nmol/kg twice daily) or vehicle. (b–g) Histomorphometric analysis shows that ascorbate prevented ovariectomy-induced bone loss (b). Indices of bone resorption (c–e) (osteoclast number per millimeter of bone surface; percentage of bone surface covered by osteoclasts; percentage of bone surface that shows scalloped, eroded appearance) were increased by ovariectomy and suppressed by ascorbate. Ascorbate also normalized (f and g) the number of osteoblasts per millimeter of bone surface and the percentage of bone surface covered by osteoblasts to sham levels. Total glutathione in bone marrow fell significantly (P < 0.05) from 32 ± 5 nmol/mg protein in controls to 22 ± 1.2 nmol/mg protein in ovariectomized mice and was increased by ascorbate to 54 ± 12.3 nmol/mg protein. (h–m) NAC (100 mg/kg/day) prevents ovariectomy-induced bone loss in mice. Indices of bone resorption and bone formation show that while ovariectomy caused a reduction in bone volume (h), this was prevented by NAC. NAC also normalized the number of osteoclasts per millimeter of the bone surface (i), the percentage of bone surface that was covered by osteoclasts (j), and the percentage of bone surface that showed a crenated, eroded surface characteristic of osteoclastic activity (k). NAC also reversed the ovariectomy-induced increase in osteoblast numbers (l) and the percentage of surface that was covered by osteoblasts (m). *P < 0.05 versus other groups. Data expressed as mean ± SEM. Oc, osteoclast; ES/BS(%), percentage of bone surface that shows an eroded surface appearance; Ob, osteoblast.
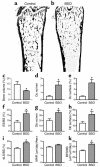
Figure 3
BSO induces bone loss in mice. (a and b) Representative sections of femora from a control mouse and a mouse injected with BSO (2 mmol/kg) twice a day for 3 weeks, showing loss of trabecular bone in BSO-treated mouse. (c) BSO caused substantial and significant loss of bone. (d–f) Bone loss was accompanied by an increase in the number of osteoclasts per millimeter of bone surface, the percentage of bone surface covered by osteoclasts, and the percentage of bone surface that showed an eroded surface. (g and h) BSO also significantly increased the number of osteoblasts covering bone surfaces and the percentage of bone surface covered by osteoblasts. (i–k) Dynamic parameters of bone formation: BSO caused a significant increase in the percentage of bone surface that was actively forming bone matrix, as judged by the percentage of surface that incorporated double calcein labels (i). MAR (mineral apposition rate, measured as the distance of separation of the double labels) was not significantly increased (j). There was an overall increase in the quantity of bone formed per unit of time (bone formation rate per unit of bone surface) (k). *P < 0.05 versus vehicle-injected control; n = 6 mice per group. Data expressed as mean ± SEM. Total glutathione fell significantly (P < 0.01) in the bone marrow of BSO-treated mice from 14.5 ± 1.1 to 6.8 ± 0.1 nmol/mg protein. There was no significant change in body weight in either group of mice during the experimental period. Uterine weights of BSO-treated mice did not differ significantly from those of control mice. Oc, osteoclast; ES/BS (%), percentage of bone surface that shows an eroded surface appearance; Ob, osteoblast; dLS/BS, proportion of bone surface that shows fluorochrome double-labeling; BFR/BS, bone formation rate expressed as the volume of bone formed per unit of bone surface.
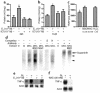
Figure 4
17-β estradiol modulates osteoclastic thiol antioxidant system, and osteoclasts are influenced by modulation of thiol antioxidant system. (a and b) 17-β estradiol stimulated glutathione reductase (GR) and thioredoxin reductase (TrxR) in osteoclasts. This stimulation was reversed by ICI 182,780 (ICI) (three cultures per variable). Statistically, significant stimulation of GR and TrxR by 17-β estradiol (E2) and inhibition of stimulation by ICI 182,780 was observed in each of two further experiments. (c) BSO stimulated TRAP-positive multinucleate cell formation (TRAP-pos MNC), while this was suppressed by NAC. Like BSO, the ROS hydrogen peroxide also stimulated TRAP-positive multinucleate cell formation (10 cultures per variable). (d) EMSA was used to show effect of BSO and NAC on NF-κB activity in in vitro–formed osteoclasts. Activity (arrowheads) was increased by BSO and suppressed by NAC. We confirmed that the bound material contained NF-κB p50 by supershifting both bands with p50 Ab (arrow). The binding was further confirmed to be specific by competing binding of NF-κB with 100-fold excess of unlabeled self probe (S), but not by a mutant species (H). M, M-CSF; RL, RANKL. (e and f) 17-β estradiol and NAC suppress expression of RNA for TNF-α in in vitro-derived osteoclasts. *P < 0.05 versus other groups.
Similar articles
- Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation.
Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Lean JM, et al. Endocrinology. 2005 Feb;146(2):728-35. doi: 10.1210/en.2004-1021. Epub 2004 Nov 4. Endocrinology. 2005. PMID: 15528306 - Tumor necrosis factor-alpha mediates osteopenia caused by depletion of antioxidants.
Jagger CJ, Lean JM, Davies JT, Chambers TJ. Jagger CJ, et al. Endocrinology. 2005 Jan;146(1):113-8. doi: 10.1210/en.2004-1058. Epub 2004 Sep 23. Endocrinology. 2005. PMID: 15388652 - Heat shock protein 60 causes osteoclastic bone resorption via toll-like receptor-2 in estrogen deficiency.
Koh JM, Lee YS, Kim YS, Park SH, Lee SH, Kim HH, Lee MS, Lee KU, Kim GS. Koh JM, et al. Bone. 2009 Oct;45(4):650-60. doi: 10.1016/j.bone.2009.06.007. Epub 2009 Jun 13. Bone. 2009. PMID: 19527807 - Estrogen, cytokines, and the control of osteoclast formation and bone resorption in vitro and in vivo.
Manolagas SC, Jilka RL, Girasole G, Passeri G, Bellido T. Manolagas SC, et al. Osteoporos Int. 1993;3 Suppl 1:114-6. doi: 10.1007/BF01621882. Osteoporos Int. 1993. PMID: 8461536 Review. No abstract available. - Estrogen regulation of immune cell bone interactions.
Weitzmann MN, Pacifici R. Weitzmann MN, et al. Ann N Y Acad Sci. 2006 Apr;1068:256-74. doi: 10.1196/annals.1346.030. Ann N Y Acad Sci. 2006. PMID: 16831927 Review.
Cited by
- Uric acid and bone mineral density in postmenopausal osteoporotic women: the link lies within the fat.
Pirro M, Mannarino MR, Bianconi V, De Vuono S, Sahebkar A, Bagaglia F, Franceschini L, Scarponi AM, Mannarino E, Merriman T. Pirro M, et al. Osteoporos Int. 2017 Mar;28(3):973-981. doi: 10.1007/s00198-016-3792-3. Epub 2016 Oct 10. Osteoporos Int. 2017. PMID: 27725998 - Effects of Low-Dose versus High-Dose γ-Tocotrienol on the Bone Cells Exposed to the Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis.
Abd Manan N, Mohamed N, Shuid AN. Abd Manan N, et al. Evid Based Complement Alternat Med. 2012;2012:680834. doi: 10.1155/2012/680834. Epub 2012 Aug 21. Evid Based Complement Alternat Med. 2012. PMID: 22956976 Free PMC article. - Suppression of autophagy in osteocytes mimics skeletal aging.
Onal M, Piemontese M, Xiong J, Wang Y, Han L, Ye S, Komatsu M, Selig M, Weinstein RS, Zhao H, Jilka RL, Almeida M, Manolagas SC, O'Brien CA. Onal M, et al. J Biol Chem. 2013 Jun 14;288(24):17432-40. doi: 10.1074/jbc.M112.444190. Epub 2013 May 3. J Biol Chem. 2013. PMID: 23645674 Free PMC article. - Myrislignan targets extracellular signal-regulated kinase (ERK) and modulates mitochondrial function to dampen osteoclastogenesis and ovariectomy-induced osteoporosis.
Yang T, Chen W, Gan K, Wang C, Xie X, Su Y, Lian H, Xu J, Zhao J, Liu Q. Yang T, et al. J Transl Med. 2023 Nov 22;21(1):839. doi: 10.1186/s12967-023-04706-2. J Transl Med. 2023. PMID: 37993937 Free PMC article. - Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids.
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Almeida M, et al. J Biol Chem. 2007 Sep 14;282(37):27285-27297. doi: 10.1074/jbc.M702810200. Epub 2007 Jul 10. J Biol Chem. 2007. PMID: 17623659 Free PMC article.
References
- Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002;23:279–302. - PubMed
- Parikka V, et al. Estrogen reduces the depth of resorption pits by disturbing the organic bone matrix degradation activity of mature osteoclasts. Endocrinology. 2001;142:5371–5378. - PubMed
- Srivastava S, et al. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-kappa B ligand (RANKL)-induced JNK activation. J. Biol. Chem. 2001;276:8836–8840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical