Chronic stress and obesity: a new view of "comfort food" - PubMed (original) (raw)
Chronic stress and obesity: a new view of "comfort food"
Mary F Dallman et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The effects of adrenal corticosteroids on subsequent adrenocorticotropin secretion are complex. Acutely (within hours), glucocorticoids (GCs) directly inhibit further activity in the hypothalamo-pituitary-adrenal axis, but the chronic actions (across days) of these steroids on brain are directly excitatory. Chronically high concentrations of GCs act in three ways that are functionally congruent. (i) GCs increase the expression of corticotropin-releasing factor (CRF) mRNA in the central nucleus of the amygdala, a critical node in the emotional brain. CRF enables recruitment of a chronic stress-response network. (ii) GCs increase the salience of pleasurable or compulsive activities (ingesting sucrose, fat, and drugs, or wheel-running). This motivates ingestion of "comfort food." (iii) GCs act systemically to increase abdominal fat depots. This allows an increased signal of abdominal energy stores to inhibit catecholamines in the brainstem and CRF expression in hypothalamic neurons regulating adrenocorticotropin. Chronic stress, together with high GC concentrations, usually decreases body weight gain in rats; by contrast, in stressed or depressed humans chronic stress induces either increased comfort food intake and body weight gain or decreased intake and body weight loss. Comfort food ingestion that produces abdominal obesity, decreases CRF mRNA in the hypothalamus of rats. Depressed people who overeat have decreased cerebrospinal CRF, catecholamine concentrations, and hypothalamo-pituitary-adrenal activity. We propose that people eat comfort food in an attempt to reduce the activity in the chronic stress-response network with its attendant anxiety. These mechanisms, determined in rats, may explain some of the epidemic of obesity occurring in our society.
Figures
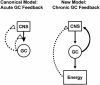
Fig. 1.
Models representing the acute and chronic effects of GC on function in the HPA axis. The canonical effects occur rapidly, within minutes to a few hours after stress; GCs act directly on brain and pituitary probably through nongenomic mechanisms. The new model requires ≈24 h, after elevation of GC into stress concentrations. Then, the direct action of GCs on brain is stimulatory, and the negative feedback inhibition of function in the HPA axis is a consequence of metabolic effects of GC increasing abdominal energy stores.
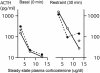
Fig. 2.
In rats exposed to a chronic stressor, high GC concentrations are required to stimulate ACTH responses to novel stimuli. Adrenalectomized rats were treated with B pellets and were maintained at room temperature (solid line, open symbol) or in cold for the next 5 days (dashed line, filled symbol). Blood was sampled in the morning within 1 min (Left) or 30 min after the onset of restraint (Right; ref. 3).
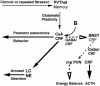
Fig. 3.
Minimal working model of the chronic stress-response network. This model is based on structures that exhibited increased numbers of c-Foslabeled cells in response to acute, novel restraint in rats with previous cold exposures compared to naive rats (6). PVThal, paraventricular nuclei of the thalamus; CeA, central nuclei of the amygdala; BNST, bed nuclei of the stria terminalis; NE, norepinephrine. Solid lines and arrows are stimulatory; dashed lines and open arrows are inhibitory.
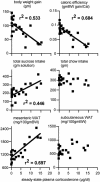
Fig. 4.
B redistributes energy stores into intraabdominal sites and increases sucrose appetite. Adrenalectomized rats were replaced with a variety of doses of B and allowed to drink sucrose for a total of 9 days in a 15-day experiment (32). Significant linear regressions between B and the variable plotted are indicated by lines and _r_2 values. Although high B concentrations strongly reduce both body weight gain and caloric efficiency, they increase both sucrose ingestion and mesenteric white adipose tissue (WAT) stores and have no effect on chow intake and s.c. WAT stores.
Fig. 5.
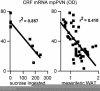
Both the amount of ingested sucrose and mesenteric WAT are significantly, negatively correlated with CRF mRNA in the PVN. All points are from adrenalectomized rats without B that were given either sucrose or saccharin. The sucrose data are from refs. and , and the mesenteric WAT results are from refs. and .
Fig. 6.
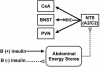
Minimal working model of the actions of B on metabolic feedback of CRF and ACTH secretion. In the presence of food intake and insulin secretion, B stimulates accretion of abdominal energy depots. By contrast, without adequate food intake and insulin secretion, there is loss of energy stores. A signal of abdominal energy stores (to date unidentified) acts to inhibit noradrenergic (A2) and adrenergic (C2) norepinephrine (NE)- or epinephrine (E)-synthesizing neurons in the nucleus of the tractus solitarius (NTS). Catecholaminergic neurons innervate all three CRF-containing structures, the central nuclei of the amygdala (CeA), the bed nuclei of the stria terminalis (BNST), and the hypothalamic PVN.
Fig. 7.
B increases salience of the pleasurable drink, saccharin. Sham-operated or adrenalectomized rats with varying B treatments were allowed to drink saccharin for 9 days in a 15-day experiment. The data shown represent drinking on the last day of the experiment (38).
Similar articles
- Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress.
Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Pecoraro N, et al. Endocrinology. 2004 Aug;145(8):3754-62. doi: 10.1210/en.2004-0305. Epub 2004 May 13. Endocrinology. 2004. PMID: 15142987 - Consequences of prenatal morphine exposure on the hypothalamo-pituitary-adrenal axis in the newborn rat: effect of maternal adrenalectomy.
Lesage J, Grino M, Bernet F, Dutriez-Casteloot I, Montel V, Dupouy JP. Lesage J, et al. J Neuroendocrinol. 1998 May;10(5):331-42. J Neuroendocrinol. 1998. PMID: 9663647 - The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons.
Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. Dallman MF, et al. Ann N Y Acad Sci. 1995 Dec 29;771:730-42. doi: 10.1111/j.1749-6632.1995.tb44724.x. Ann N Y Acad Sci. 1995. PMID: 8597446 Review. - Activation of the hypothalamic-pituitary axis in adrenalectomised rats: potentiation by chronic stress.
Martí O, Harbuz MS, Andrés R, Lightman SL, Armario A. Martí O, et al. Brain Res. 1999 Mar 6;821(1):1-7. doi: 10.1016/s0006-8993(98)01212-8. Brain Res. 1999. PMID: 10064781 - Chronic stress and comfort foods: self-medication and abdominal obesity.
Dallman MF, Pecoraro NC, la Fleur SE. Dallman MF, et al. Brain Behav Immun. 2005 Jul;19(4):275-80. doi: 10.1016/j.bbi.2004.11.004. Brain Behav Immun. 2005. PMID: 15944067 Review.
Cited by
- Improvements in Neighborhood Socioeconomic Conditions May Improve Resident Diet.
Richardson AS, Collins RL, Ghosh-Dastidar B, Ye F, Hunter GP, Baird MD, Schwartz H, Sloan JC, Nugroho A, Beckman R, Troxel WM, Gary-Webb TL, Dubowitz T. Richardson AS, et al. Am J Epidemiol. 2021 May 4;190(5):798-806. doi: 10.1093/aje/kwaa220. Am J Epidemiol. 2021. PMID: 33047782 Free PMC article. - Activating Corticotropin-Releasing Factor Systems in the Nucleus Accumbens, Amygdala, and Bed Nucleus of Stria Terminalis: Incentive Motivation or Aversive Motivation?
Baumgartner HM, Schulkin J, Berridge KC. Baumgartner HM, et al. Biol Psychiatry. 2021 Jun 15;89(12):1162-1175. doi: 10.1016/j.biopsych.2021.01.007. Epub 2021 Jan 21. Biol Psychiatry. 2021. PMID: 33726937 Free PMC article. - Stress eating and health. Findings from MIDUS, a national study of US adults.
Tsenkova V, Boylan JM, Ryff C. Tsenkova V, et al. Appetite. 2013 Oct;69:151-5. doi: 10.1016/j.appet.2013.05.020. Epub 2013 Jun 6. Appetite. 2013. PMID: 23747576 Free PMC article. - Lack of stress responses to long-term effects of corticosterone in Caps2 knockout mice.
Mishima Y, Shinoda Y, Sadakata T, Kojima M, Wakana S, Furuichi T. Mishima Y, et al. Sci Rep. 2015 Mar 10;5:8932. doi: 10.1038/srep08932. Sci Rep. 2015. PMID: 25754523 Free PMC article. - Irregularities in glucose metabolism induced by stress and high-calorie diet can be attenuated by glycyrrhizic acid.
Yaw HP, Ton SH, Amanda S, Kong IG, Cheng HS, Fernando HA, Chin HF, Kadir KA. Yaw HP, et al. Int J Physiol Pathophysiol Pharmacol. 2014 Dec 15;6(4):172-84. eCollection 2014. Int J Physiol Pathophysiol Pharmacol. 2014. PMID: 25755839 Free PMC article.
References
- Keller-Wood, M. E. & Dallman, M. F. (1984) Endocr. Rev. 5, 1–24. - PubMed
- Buwalda, B., De Boer, S. F., Schmidt, E. D., Felszeghy, K., Nyaka, C., Sgoigo, A., Van der Begt, B. J., Tilders, F. H. J., Bohus, B. & Koolhaas, J. M. (1999) J. Neuroendocrinol. 11, 512–520. - PubMed
- Akana, S. F. & Dallman, M. F. (1997) Endocrinology 138, 3249–3258. - PubMed
- Young, E. A., Kwak, S. P. & Kottak, J. (1995) J. Neuroendocrinol. 7, 37–45. - PubMed
- Kuipers, S. D., Trentani, A., den Boer, J. A. & Ter Horst, G. J. (2003) J. Neurochem. 85, 1312–1323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 DA014159/DA/NIDA NIH HHS/United States
- F32-DA14159/DA/NIDA NIH HHS/United States
- F32-DA14143/DA/NIDA NIH HHS/United States
- R01 DK028172/DK/NIDDK NIH HHS/United States
- R37 DK028172/DK/NIDDK NIH HHS/United States
- F32 DA014143/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous