Gating and regulation of connexin 43 (Cx43) hemichannels - PubMed (original) (raw)
Gating and regulation of connexin 43 (Cx43) hemichannels
Jorge E Contreras et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Connexin 43 (Cx43) nonjunctional or "unapposed" hemichannels can open under physiological or pathological conditions. We characterize hemichannels comprised of Cx43 or Cx43-EGFP (Cx43 with enhanced GFP fused to the C terminus) expressed in HeLa cells. Channel opening was induced at potentials greater than +60 mV. Open probability appeared to be very low. No comparable opening was detected in the parental, nontransfected HeLa cells. Conductance of fully open single hemichannels was approximately 220 pS, which is approximately double that of Cx43 cell-cell channels. Cx43 hemichannels exhibited two types of gating: fast transitions (<1 ms) between the fully open state and a substate of approximately 75 pS and slow transitions (>5 ms) between either open state and the fully closed state. Cx43-EGFP hemichannels exhibited only slow transitions (>5 ms) between closed and fully open states. These properties resemble those of the corresponding Cx43 and Cx43-EGFP cell-cell channels. Cx43 with EGFP on the N terminus (EGFP-Cx43) inserted into the surface and formed plaques but did not form hemichannels or cell-cell channels. Hemichannel blockers, 18beta-glycyrrhetinic acid or La3+, blocked depolarization-induced currents. Uptake of ethidium bromide (i) was faster in Cx43 and Cx43-EGFP than parental and EGFP-Cx43 cells, (ii) was directly correlated with Cx43-EGFP expression, (iii) was reduced by hemichannel blockers, and (iv) occurred at the same low rate in EGFP-Cx43 and parental cells. Although hemichannel opening was not detected electrophysiologically at the resting potential, infrequent or brief opening could account for ethidium bromide uptake. Opening of Cx43 hemichannels may mediate normal signaling or be deleterious.
Figures
Fig. 1.
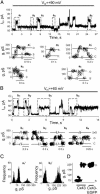
Positive voltages induce hemichannel currents in HeLa cells expressing Cx43 and Cx43-EGFP but not in parental cells. (A) Voltage ramps from –110 to +110mV, 6sin duration separated by 1 s, were applied; the zero potential was the resting potential. (B_–_D) Currents of parental, Cx43, and Cx43-EGFP cells, respectively. Large increases in current were evoked at voltages greater than +60 mV in Cx43 and Cx43-EGFP cells (C and D), but only small increases were evoked in parental cells (B). All cells show small increases in conductance at large negative potentials. Expanded records are shown for the ramps in B and C ending at 28 s. Presumptive single-channel openings are indicated by dotted lines. Open channels closed by the beginning of the next ramp except for the ramp ending at 21 s (D). Records in this figure are representative of 15 cells of each type.
Fig. 2.
Unitary conductance and voltage gating of Cx43 and Cx43-EGFP hemichannels. (A Upper) Single-channel events in a Cx43 cell at a holding _V_m of +90 mV. Two channels were fully open just after 18 s. (Lower) Boxed regions in Upper are shown as conductances on an expanded time scale (reversal potential assumed to be 0; leakage current = 52 pA; open circles indicate 2-ms time points). a1, A single channel opened at 1 s to a conductance of ≈220 pS and then closed to a substate of ≈75 pS. The opening was slow (>10 ms), but the closure to the substate was fast and not resolved. a2, Slow opening to the substate. a3, Slow opening and closing of a second channel superimposed on the substate of the first channel (redefined as zero current here). a4, Slow closing of the substate of the first channel. a5, Fast opening and closing of one channel superimposed on the substate of both channels. O, open state; S, substate; C, closed state. (B) Single-channel activity in a Cx43-EGFP cell; a maximum of two channels were open simultaneously. The display is as described for A.b1–b4, Records with higher time resolution showing only slow transitions between open and closed states. (C) All-points histograms from a1 and b3 showing three peaks for Cx43 and two for Cx43-EGFP. (D) Conductances of substate and main state; each point is the mean of several measurements from a different cell. The main state had the same conductance for Cx43 (220 ± 11 pS; n = 10) and Cx43-EGFP (223 ± 9 pS; n = 15). The conductance of the Cx43 substate was 77 ± 13 pS (n = 10).
Fig. 3.
EGFP-Cx43 in HeLa cells reaches the cell surface and forms plaques but no functional hemichannels or channels. (A) A pair of EGFP-Cx43 cells forms a fluorescent plaque typical of a gap junction (arrow). (B) Application of voltage ramps (Lower) to cell 1 did not induce junctional currents in the other cell (Upper). (C) Phase. (D) Fluorescence of an isolated EGFP-Cx43 cell shows expression at the surface membrane. (E) Two records of polarization to +90 mV show no Cx43-like single-channel activity (an ≈20-pA step would be seen after opening of a 220-pS Cx43 channel; see Fig. 2).
Fig. 4.
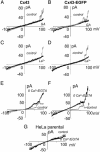
Hemichannel opening is blocked by gap junction blockers and potentiated by zero Ca2+/EGTA. (B, D, and F) Cx43 cells. (A, C, and E) Cx43-EGFP cells. –/+ voltage ramps were applied as described for Fig. 1. (A and B) α-Glycyrrhetinic acid (GA, 35 μM) blocked channel opening at positive potentials with little effect on resting conductance. (C and D) La3+ also blocked hemichannel currents with little effect on resting conductance. (E and F) Zero Ca2+/EGTA (2 mM) increased opening at positive potentials, and occasionally channels remained open between ramps (F), but there was little effect on resting conductance. (G) Zero Ca2+/EGTA had little effect on parental cells over the entire voltage range.
Fig. 5.
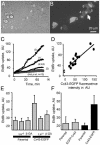
EtdBr uptake by Cx43-EGFP cells in standard culture conditions is mediated by opening of hemichannels. (A) Phase contrast of a mixed culture of parental and Cx43-EGFP cells seeded 16 h before dye uptake assay. (B) Cx43-EGFP fluorescence of the field shown in A; six cells expressing different levels of EGFP are numbered 1–6. Cells 4–6 are in contact and show fluorescent gap junction plaques between them (arrows). The rounded-up cells on the left are dividing parental cells; flattened interphase parental cells are also present. (C) Cells were incubated in 10 μM EtdBr, and dye uptake was measured every 45 s as fluorescence emission of EtdBr binding to DNA [518 nm, arbitrary units (AU) of intensity]. Uptake was greatest in cell 3, which exhibited the most EGFP fluorescence, and less for the other cells. Uptake for parental cells (n = 15) was less than for any of the Cx43-EGFP cells. (D) Plot of EtdBr fluorescence (at 60 min) as a function of Cx43-EGFP fluorescence for 13 cells and for mean uptake by parental cells (n = 15). Uptake was linearly related to EGFP fluorescence (r = 0.79 for Cx43-EGFP cells). (E) Gap junction blockers La3+ (0.1 mM) and 18β-glycyrrhetinic acid (GA, 35 μM) reduced EtdBr uptake by Cx43-EGFP cells (P < 0.05 for parental vs. Cx43-EGFP, other pairings not significant by _t_ test) but did not affect uptake by parental cells. (_F_) Dye uptake after a 75-min incubation with 10 μM EtdBr in mixed cultures of parental cells with EGFP-Cx43 cells (gray bars) or mixed cultures of parental with Cx43-EGFP cells (black bars). EtdBr uptake was low and not significantly different in EGFP-Cx43 and parental cells (_P_ > 0.3, n = 20 for each cell type); EtdBr uptake was greater in Cx43-EGFP than in parental cells (P < 0.05, n = 20 for each cell type).
Similar articles
- Functioning of cx43 hemichannels demonstrated by single channel properties.
Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. Contreras JE, et al. Cell Commun Adhes. 2003 Jul-Dec;10(4-6):245-9. doi: 10.1080/cac.10.4-6.245.249. Cell Commun Adhes. 2003. PMID: 14681024 - Coupling asymmetry of heterotypic connexin 45/ connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms.
Bukauskas FF, Angele AB, Verselis VK, Bennett MV. Bukauskas FF, et al. Proc Natl Acad Sci U S A. 2002 May 14;99(10):7113-8. doi: 10.1073/pnas.032062099. Proc Natl Acad Sci U S A. 2002. PMID: 12011467 Free PMC article. - Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential.
Retamal MA, Schalper KA, Shoji KF, Bennett MV, Sáez JC. Retamal MA, et al. Proc Natl Acad Sci U S A. 2007 May 15;104(20):8322-7. doi: 10.1073/pnas.0702456104. Epub 2007 May 9. Proc Natl Acad Sci U S A. 2007. PMID: 17494739 Free PMC article. - Emerging issues of connexin channels: biophysics fills the gap.
Harris AL. Harris AL. Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review. - Connexin-based gap junction hemichannels: gating mechanisms.
Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Sáez JC, et al. Biochim Biophys Acta. 2005 Jun 10;1711(2):215-24. doi: 10.1016/j.bbamem.2005.01.014. Epub 2005 Mar 2. Biochim Biophys Acta. 2005. PMID: 15955306 Free PMC article. Review.
Cited by
- Manipulating connexin communication channels: use of peptidomimetics and the translational outputs.
Evans WH, Bultynck G, Leybaert L. Evans WH, et al. J Membr Biol. 2012 Aug;245(8):437-49. doi: 10.1007/s00232-012-9488-5. Epub 2012 Aug 11. J Membr Biol. 2012. PMID: 22886208 Free PMC article. Review. - Cryo-EM structure of the heptameric calcium homeostasis modulator 1 channel.
Ren Y, Li Y, Wang Y, Wen T, Lu X, Chang S, Zhang X, Shen Y, Yang X. Ren Y, et al. J Biol Chem. 2022 May;298(5):101838. doi: 10.1016/j.jbc.2022.101838. Epub 2022 Mar 24. J Biol Chem. 2022. PMID: 35339491 Free PMC article. - Intracellular calcium changes trigger connexin 32 hemichannel opening.
De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. De Vuyst E, et al. EMBO J. 2006 Jan 11;25(1):34-44. doi: 10.1038/sj.emboj.7600908. Epub 2005 Dec 8. EMBO J. 2006. PMID: 16341088 Free PMC article. - Pannexin 1 in erythrocytes: function without a gap.
Locovei S, Bao L, Dahl G. Locovei S, et al. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7655-9. doi: 10.1073/pnas.0601037103. Epub 2006 May 8. Proc Natl Acad Sci U S A. 2006. PMID: 16682648 Free PMC article. - Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit.
Fellin T, Carmignoto G. Fellin T, et al. J Physiol. 2004 Aug 15;559(Pt 1):3-15. doi: 10.1113/jphysiol.2004.063214. Epub 2004 Jun 24. J Physiol. 2004. PMID: 15218071 Free PMC article. Review.
References
- Hofer, A. & Dermietzel, R. (1998) Glia 24, 141–154. - PubMed
- John, S. A., Kondo, R., Wang, S. Y., Goldhaber, J. I. & Weiss. J. N. (1999) J. Biol. Chem. 274, 236–240. - PubMed
- Valiunas, V. & Weingart, R. (2000) Pflügers Arch. 440, 366–379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources