Expression of Epstein-Barr virus-encoded small RNA (by the EBER-1 gene) in liver specimens from transplant recipients with post-transplantation lymphoproliferative disease - PubMed (original) (raw)
Expression of Epstein-Barr virus-encoded small RNA (by the EBER-1 gene) in liver specimens from transplant recipients with post-transplantation lymphoproliferative disease
P S Randhawa et al. N Engl J Med. 1992.
Abstract
Background: Epstein-Barr virus (EBV)-associated post-transplantation lymphoproliferative disease (PTLD) develops in 1 to 10 percent of transplant recipients, in whom it can be treated by a reduction in the level of immunosuppression. We postulated that the tissue expression of the small RNA transcribed by the EBER-1 gene during latent EBV infection would identify patients at risk for PTLD.
Methods: We studied EBER-1 gene expression in liver specimens obtained from 24 patients 2 days to 22 months before the development of PTLD, using in situ hybridization with an oligonucleotide probe. Control specimens were obtained from 20 recipients of allografts with signs of injury due to organ retrieval, acute graft rejection, or viral hepatitis in whom PTLD had not developed 9 to 71 months after the biopsy.
Results: Of the 24 patients with PTLD, 17 (71 percent) had specimens in which 1 to 40 percent of mononuclear cells were positive for the EBER-1 gene. In addition, 10 of these 17 patients (59 percent) had specimens with histopathological changes suggestive of EBV hepatitis. In every case, EBER-1-positive cells were found within the lymphoproliferative lesions identified at autopsy. Only 2 of the 20 controls (10 percent) had specimens with EBER-1-positive cells (P < 0.001), and such cells were rare.
Conclusions: EBER-1 gene expression in liver tissue precedes the occurrence of clinical and histologic PTLD. The possibility of identifying patients at risk by the method we describe here and preventing the occurrence of PTLD by a timely reduction of immunosuppression needs to be addressed by future prospective studies.
Figures
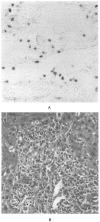
Figure 1
Lymphocytic Infiltration of the Hepatic Portal Tracts by Mononuclear cells Positive for EBER-1 mRNA in a Patient with EBV-Associated Hepatitis. Panel A (×210) shows scattered EBER-1–positive nuclei in approximately 20 percent of the lymphocytes infiltrating the portal tract. Panel B (hematoxylin and eosin, ×210) shows an infiltrate of activated lymphocytes involving the portal vein and simulating graft rejection.
Figure 2
Lymphocytic Infiltration of the Hepatic Sinusoids by EBER-1–Positive Cells in a Patient with EBV-Associated Hepatitis. In Panel A (×210), the sinusoids contain aggregates of lymphocytes, and the hepatocytes show focal hydropic swelling, steatosis, and nuclear pyknosis. In Panel B (×210), the sinusoids also contain EBER-1–positive lymphocytes.
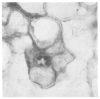
Figure 3
PTLD Involving a Portal Tract (× 420). Approximately 70 percent of the cells are EBER-1–positive, with both the small round lymphocytes and the large immature lymphocytes affected. The bile-duct epithelium in the center of the field is stained with anti–Cam 5.2 antibody and does not show evidence of EBV infection.
Figure 4
Fatal PTLD Involving Hepatocytes (×630). Rare EBER-1–positive nuclei were found In cells conclusively identified as hepatocytes, by simultaneous immunohistochemical evaluation with the anti-keratin antibody Cam 5. 2.
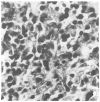
Figure 5
EBV-Infected Lymphocytes in the Lesions of PTLD (×420). Immunohistochemical evaluation with anti-CD20 antibody, combined with in situ hybridization, showed that in PTLD the majority of EBV-infected lymphocytes are B cells. Cells positive for CD20 are stained brown, and those positive for EBER-1 mRNA are stained blue-black.
Comment in
- Epstein-Barr virus: culprit or consort?
Pagano JS. Pagano JS. N Engl J Med. 1992 Dec 10;327(24):1750-2. doi: 10.1056/NEJM199212103272410. N Engl J Med. 1992. PMID: 1331791 No abstract available. - Expression of Epstein-Barr virus in liver-transplant recipients with lymphoproliferative disease.
Francis JB. Francis JB. N Engl J Med. 1993 Jul 15;329(3):208; author reply 208-9. doi: 10.1056/NEJM199307153290315. N Engl J Med. 1993. PMID: 8390617 No abstract available.
Similar articles
- Posttransplant lymphoproliferative disorder in liver allograft biopsies: a comparison of three methods for the demonstration of Epstein-Barr virus.
Lones MA, Shintaku IP, Weiss LM, Thung SN, Nichols WS, Geller SA. Lones MA, et al. Hum Pathol. 1997 May;28(5):533-9. doi: 10.1016/s0046-8177(97)90074-5. Hum Pathol. 1997. PMID: 9158700 - Association between Epstein-Barr virus seroconversion and immunohistochemical changes in tonsils of pediatric solid organ transplant recipients.
Shapiro NL, Tang CG, Bhattacharyya N. Shapiro NL, et al. Laryngoscope. 2011 Aug;121(8):1718-25. doi: 10.1002/lary.21871. Laryngoscope. 2011. PMID: 21792960 - Epstein-Barr virus infections in children after transplantation of the small intestine.
Finn L, Reyes J, Bueno J, Yunis E. Finn L, et al. Am J Surg Pathol. 1998 Mar;22(3):299-309. doi: 10.1097/00000478-199803000-00004. Am J Surg Pathol. 1998. PMID: 9500771 - Epstein-Barr virus-related localized hepatic lymphoproliferative disorders after liver transplantation.
Raymond E, Tricottet V, Samuel D, Reynès M, Bismuth H, Misset JL. Raymond E, et al. Cancer. 1995 Oct 15;76(8):1344-51. doi: 10.1002/1097-0142(19951015)76:8<1344::aid-cncr2820760808>3.0.co;2-k. Cancer. 1995. PMID: 8620407 Review.
Cited by
- Lung transplantation for cystic fibrosis: results, indications, complications, and controversies.
Lynch JP 3rd, Sayah DM, Belperio JA, Weigt SS. Lynch JP 3rd, et al. Semin Respir Crit Care Med. 2015 Apr;36(2):299-320. doi: 10.1055/s-0035-1547347. Epub 2015 Mar 31. Semin Respir Crit Care Med. 2015. PMID: 25826595 Free PMC article. Review. - The molecular genetics of post-transplantation lymphoproliferative disorders.
Knowles DM. Knowles DM. Springer Semin Immunopathol. 1998;20(3-4):357-73. doi: 10.1007/BF00838049. Springer Semin Immunopathol. 1998. PMID: 9870251 Review. - Detection of Epstein-Barr virus DNA in sera from transplant recipients with lymphoproliferative disorders.
Limaye AP, Huang ML, Atienza EE, Ferrenberg JM, Corey L. Limaye AP, et al. J Clin Microbiol. 1999 Apr;37(4):1113-6. doi: 10.1128/JCM.37.4.1113-1116.1999. J Clin Microbiol. 1999. PMID: 10074534 Free PMC article. - Virus-Driven Carcinogenesis.
Hatano Y, Ideta T, Hirata A, Hatano K, Tomita H, Okada H, Shimizu M, Tanaka T, Hara A. Hatano Y, et al. Cancers (Basel). 2021 May 27;13(11):2625. doi: 10.3390/cancers13112625. Cancers (Basel). 2021. PMID: 34071792 Free PMC article. Review. - Epstein-Barr virus infection in transplant recipients: Summary of a workshop on surveillance, prevention and treatment.
Allen U, Alfieri C, Preiksaitis J, Humar A, Moore D, Tapiero B, Tellier R, Green M, Davies D, Hébert D, Weitzman S, Petric M, Jacobson K; Canadian PTLD Workshop Group - 1999. Allen U, et al. Can J Infect Dis. 2002 Mar;13(2):89-99. doi: 10.1155/2002/634318. Can J Infect Dis. 2002. PMID: 18159378 Free PMC article.
References
- Young L, Alfieri C, Hennessy K, et al. Expression of Epstein–Barr virus transformation–associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–5. - PubMed
- Thomas JA, Hotchin NA, Allday MJ, et al. Immunohistology of Epstein-Barr virus-associated antigens in B cell disorders from immunocompromised individuals. Transplantation. 1990;49:944–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical