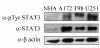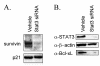Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells - PubMed (original) (raw)
Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells
Liza Konnikova et al. BMC Cancer. 2003.
Abstract
Background: Astrocytomas are the most common type of primary central nervous system tumors. They are frequently associated with genetic mutations that deregulate cell cycle and render these tumors resistant to apoptosis. STAT3, signal transducer and activator of transcription 3, participates in several human cancers by inducing cell proliferation and inhibiting apoptosis and is frequently activated in astrocytomas.
Methods: RNA interference was used to knockdown STAT3 expression in human astrocytes and astrocytoma cell lines. The effect of STAT3 knockdown on apoptosis, cell proliferation, and gene expression was then assessed by standard methods.
Results: We have found that STAT3 is constitutively activated in several human astrocytoma cell lines. Knockdown of STAT3 expression by siRNA induces morphologic and biochemical changes consistent with apoptosis in several astrocytoma cell lines, but not in primary human astrocytes. Moreover, STAT3 is required for the expression of the antiapoptotic genes survivin and Bcl-xL in the A172 glioblastoma cell line.
Conclusion: These results show that STAT3 is required for the survival of some astrocytomas. These studies suggest STAT3 siRNA could be a useful therapeutic agent for the treatment of astrocytomas.
Figures
Figure 1
STAT3 is expressed and active in GBM cell lines. The indicated cell lines were lysed in RIPA buffer after serum starvation for 24 hrs. Cell lysates were subjected to electrophoresis on an SDS PAGE gel and processed for Western blotting with anti-phospho-Tyr-STAT3, anti-STAT3, or anti-β-actin antibodies. NHA are primary human astrocytes. All other cell lines are grade IV astrocytoma (GBM).
Figure 2
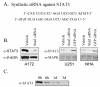
Knockdown of STAT3 by siRNA in primary human astrocytes and astrocytoma cells. A. Sequences of the synthetic siRNA duplex designed against human STAT3. B. The astrocytoma cell lines A172 and U251 and normal human astrocytes (NHA) cultured in 10 cm plates were transfected with 600 nmol STAT3 siRNA and after 72 hours processed for Western blotting with anti-STAT3 antibodies. The blots were subsequently stripped and reprobed with anti-β-actin or anti-Map Kinase antibodies as controls. C. A172 cells were transfected with 600 nmol STAT3 siRNA for the times indicated and processed for Western blotting with anti-STAT3 antibodies.
Figure 3
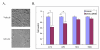
STAT3 siRNA causes a decrease in cell number in glioblastoma cell lines. A. A172 cells were transfected with a STAT3 siRNA (bottom panel) or a control siRNA (top panel). Cells were photographed 72 hours post transfection at 200× magnification. B. A172, U251, T98G, and NHA cells were transfected with STAT3 siRNA or mock transfected with oligofectamine (vehicle). Viable cell numbers were determined by MTS assay 72 hours after transfection. (*) indicates a P value <0.001.
Figure 4
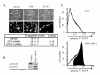
Induction of apoptosis in human astrocytomas by STAT3 RNAi. A. A172 cells were transfected with STAT3, GFP siRNAs, or mock transfected with oligofectamine (vehicle). After 72 hrs, cells were fixed in 4% paraformaldehyde and stained with 25 μg/ml Hoechst 33258 dye to visualize apoptotic nuclei. The percentage of apoptotic cells (small bright nucleus) was determined for each of the treatments by counting five visual fields and at least 100 cells. B. A172 cells were transfected with STAT3 siRNA or mock transfected with oligofectamine. Western blotting was performed 72 hrs post transfection. Blots were probed with α-STAT3, α-cleaved caspase 3 and α-β-actin antibodies. C. A172 cells were transfected with STAT3 siRNA (bottom panel) or mock transfected with oligofectamine (top panel). After 96 hours, cells were isolated and immunostained with EGFP coupled anti-annexin V antibody. Cells were sorted by FACS and annexin V positive cells counted. The region shaded in black (77%) corresponds the increase in annexin V positive cells relative to the mock transfected cells.
Figure 5
STAT3 controls expression of survivin and Bcl-xL in the A172 human glioblastoma cell line. A. A172 cells were transfected with STAT3 siRNA or mock transfected with oligofectamine. After 72 hours, RNA was isolated from the cells and then analyzed by northern blotting for survivin and p21 mRNA. B. A172 cells were transfected with STAT3 siRNA or mock transfected with oligofectamine. After 72 hrs, STAT3. β-actin, and Bcl-xL expression levels were analyzed by Western blotting.
Similar articles
- Down-regulation of signal transducer and activator of transcription 3 expression using vector-based small interfering RNAs suppresses growth of human prostate tumor in vivo.
Gao L, Zhang L, Hu J, Li F, Shao Y, Zhao D, Kalvakolanu DV, Kopecko DJ, Zhao X, Xu DQ. Gao L, et al. Clin Cancer Res. 2005 Sep 1;11(17):6333-41. doi: 10.1158/1078-0432.CCR-05-0148. Clin Cancer Res. 2005. PMID: 16144938 - Inhibition of signal transducer and activator of transcription 3 expression by RNA interference suppresses invasion through inducing anoikis in human colon cancer cells.
Fan Y, Zhang YL, Wu Y, Zhang W, Wang YH, Cheng ZM, Li H. Fan Y, et al. World J Gastroenterol. 2008 Jan 21;14(3):428-34. doi: 10.3748/wjg.14.428. World J Gastroenterol. 2008. PMID: 18200666 Free PMC article. - STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells.
Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y, Sekikawa A, Kawada M, Suzuki K, Kayahara T, Fukui H, Sawada M, Chiba T. Kanda N, et al. Oncogene. 2004 Jun 17;23(28):4921-9. doi: 10.1038/sj.onc.1207606. Oncogene. 2004. PMID: 15077160 - STAT3 silencing with lentivirus inhibits growth and induces apoptosis and differentiation of U251 cells.
Li GH, Wei H, Chen ZT, Lv SQ, Yin CL, Wang DL. Li GH, et al. J Neurooncol. 2009 Jan;91(2):165-74. doi: 10.1007/s11060-008-9696-0. Epub 2008 Oct 7. J Neurooncol. 2009. PMID: 18839277 - Inhibition of STAT3 expression by siRNA suppresses growth and induces apoptosis in laryngeal cancer cells.
Gao LF, Xu DQ, Wen LJ, Zhang XY, Shao YT, Zhao XJ. Gao LF, et al. Acta Pharmacol Sin. 2005 Mar;26(3):377-83. doi: 10.1111/j.1745-7254.2005.00053.x. Acta Pharmacol Sin. 2005. PMID: 15715937
Cited by
- Glioma revisited: from neurogenesis and cancer stem cells to the epigenetic regulation of the niche.
de Almeida Sassi F, Lunardi Brunetto A, Schwartsmann G, Roesler R, Abujamra AL. de Almeida Sassi F, et al. J Oncol. 2012;2012:537861. doi: 10.1155/2012/537861. Epub 2012 Jul 8. J Oncol. 2012. PMID: 22973309 Free PMC article. - Potential use of STAT3 inhibitors in targeted prostate cancer therapy: future prospects.
Shodeinde AL, Barton BE. Shodeinde AL, et al. Onco Targets Ther. 2012;5:119-25. doi: 10.2147/OTT.S32559. Epub 2012 Jul 4. Onco Targets Ther. 2012. PMID: 22815644 Free PMC article. - Mechanisms of malignant glioma immune resistance and sources of immunosuppression.
Gomez GG, Kruse CA. Gomez GG, et al. Gene Ther Mol Biol. 2006;10(A):133-146. Gene Ther Mol Biol. 2006. PMID: 16810329 Free PMC article. - Immunotherapy of diffuse gliomas: biological background, current status and future developments.
Grauer OM, Wesseling P, Adema GJ. Grauer OM, et al. Brain Pathol. 2009 Oct;19(4):674-93. doi: 10.1111/j.1750-3639.2009.00315.x. Brain Pathol. 2009. PMID: 19744040 Free PMC article. Review. - A novel tumor-promoting role for nuclear factor IA in glioblastomas is mediated through negative regulation of p53, p21, and PAI1.
Lee JS, Xiao J, Patel P, Schade J, Wang J, Deneen B, Erdreich-Epstein A, Song HR. Lee JS, et al. Neuro Oncol. 2014 Jan;16(2):191-203. doi: 10.1093/neuonc/not167. Epub 2013 Dec 4. Neuro Oncol. 2014. PMID: 24305710 Free PMC article.
References
- Prados MD, Levin V. Biology and treatment of malignant glioma. Semin Oncol. 2000;27:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous