Itk functions to control actin polymerization at the immune synapse through localized activation of Cdc42 and WASP - PubMed (original) (raw)
Itk functions to control actin polymerization at the immune synapse through localized activation of Cdc42 and WASP
Christine M Labno et al. Curr Biol. 2003.
Abstract
Actin polymerization at the immune synapse is required for T cell activation and effector function; however, the relevant regulatory pathways remain poorly understood. We showed previously that binding to antigen presenting cells (APCs) induces localized activation of Cdc42 and Wiskott-Aldrich Syndrome protein (WASP) at the immune synapse. Several lines of evidence suggest that Tec kinases could interact with WASP-dependent actin regulatory processes. Since T cells from Rlk-/-, Itk-/-, and Rlk-/- x Itk-/- mice have defects in signaling and development, we asked whether Itk or Rlk function in actin polymerization at the immune synapse. We find that Itk-/- and Rlk-/- x Itk-/- T cells are defective in actin polymerization and conjugate formation in response to antigen-pulsed APCs. Itk functions downstream of the TCR, since similar defects were observed upon TCR engagement alone. Using conformation-specific probes, we show that although the recruitment of WASP and Arp2/3 complex to the immune synapse proceeds normally, the localized activation of Cdc42 and WASP is defective. Finally, we find that the defect in Cdc42 activation likely stems from a requirement for Itk in the recruitment of Vav to the immune synapse. Our results identify Itk as a key element of the pathway leading to localized actin polymerization at the immune synapse.
Figures
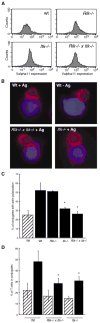
Figure 1. Actin Polymerization at the Immune Synapse and Conjugate Formation Are Reduced in Rlk−/− × Itk−/− and Itk−/− T Cells
(A) Primary T cell blasts from AND TCR Tg mice were analyzed by flow cytometry to verify comparable surface expression of the Tg TCR. (B) Primary T cell blasts were allowed to conjugate to CMAC (blue)-dyed B cells ± Ag for 10 min, were fixed, and were stained with rhodamine phalloidin to detect F-actin (red). Note the lack of F-actin enrichment at the immune synapse in conjugates formed with the mutant T cells. (C) Conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were selected at random and were scored for accumulation of F-actin at the immune synapse. Data are mean ± SD from at least 3 independent experiments, with 50 conjugates each (the asterisk indicates a significant difference from wt + Ag, p < 0.001). (D) Conjugation efficiency in the absence (hatched bars) or presence (solid bars) of Ag was analyzed by flow cytometry as described in the Experimental Procedures. Data are mean ± SD from at least three independent experiments (the asterisk indicates a significant difference from wt + Ag, p < 0.05).
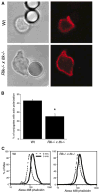
Figure 2. Defects in Actin Polymerization Occur Downstream of TCR Engagement
(A) Splenic T cells were allowed to conjugate to anti-TCR-coated beads, were fixed, and were stained with Alexa 594-phalloidin to detect F-actin (red). Note the lack of F-actin enrichment at the bead interface with the Rlk−/− × Itk−/− T cell. (B) Bead conjugates formed as in (A) were scored for accumulation of F-actin. Data are mean ± SD from six independent experiments (the asterisk indicates a significant difference from wt, p < 0.0001). (C) Splenic T cells were stimulated for 5 min by soluble anti-CD3 crosslinking and were fixed, and F-actin content was assessed after labeling with Alexa 488-phalloidin.
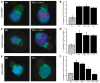
Figure 3. WASP and Arp2/3 Are Recruited to the Immune Synapse, but WASP Remains in an Inactive State
(A) Conjugates were labeled with an antibody that recognizes WASP in a conformation-independent manner. Both wt and Rlk−/− × Itk−/− T cells show recruitment of WASP to the immune synapse. (B) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for WASP polarization. (C) Conjugates were stained with an anti-Arp3 antibody. Both wt and Rlk−/− × Itk−/− T cells show accumulation of Arp2/3 complex at the interface. (D) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for Arp2/3 polarization. (E) Conjugates were stained with a monoclonal antibody that preferentially recognizes the open, activated form of WASP. Note that active WASP is not enriched at the immune synapse in the Itk−/− T cell. (F) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for accumulation of open WASP at the immune synapse. Data in (B), (D), and (F) represent means from at least three independent experiments ± SD (the asterisk indicates a significant difference from wt + Ag, p < 0.01 for Itk−/−, and p < 0.001 for Rlk−/− × Itk−/− T cells).
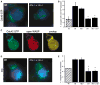
Figure 4. Itk Is Required for Vav Recruitment and Cdc42 Activation at the Immune Synapse
(A) Conjugates were labeled with recombinant GFP-WASP-GBD to detect Cdc42-GTP. Note the enrichment of activated Cdc42 at the immune synapse of the conjugate formed with the wt T cell, but not the Rlk−/− × Itk−/− T cell. (B) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for accumulation of Cdc42-GTP at the immune synapse. (C) Double labeling was performed by using the GFP-WASP-GBD reagent to detect Cdc42-GTP (green) and mAb 26E6 to detect the open, active conformation of WASP (red). The overlaid image shows extensive colocalization of activated Cdc42 and WASP at the immune synapse (yellow). (D) Conjugates were labeled with anti-Vav antibody. Note the enrichment of Vav at the immune synapse of the conjugate formed with the wt T cell, but not the Rlk−/− × Itk−/− T cell. (E) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for accumulation of Vav at the immune synapse. Data in (B) and (F) represent means from at least three independent experiments ± SD (the asterisk indicates a significant difference from wt + Ag, p < 0.001).
Similar articles
- SLP-76 coordinates Nck-dependent Wiskott-Aldrich syndrome protein recruitment with Vav-1/Cdc42-dependent Wiskott-Aldrich syndrome protein activation at the T cell-APC contact site.
Zeng R, Cannon JL, Abraham RT, Way M, Billadeau DD, Bubeck-Wardenberg J, Burkhardt JK. Zeng R, et al. J Immunol. 2003 Aug 1;171(3):1360-8. doi: 10.4049/jimmunol.171.3.1360. J Immunol. 2003. PMID: 12874226 - The regulation of actin remodeling during T-cell-APC conjugate formation.
Cannon JL, Burkhardt JK. Cannon JL, et al. Immunol Rev. 2002 Aug;186:90-9. doi: 10.1034/j.1600-065x.2002.18609.x. Immunol Rev. 2002. PMID: 12234365 Review. - Wiskott-Aldrich syndrome protein--dynamic regulation of actin homeostasis: from activation through function and signal termination in T lymphocytes.
Matalon O, Reicher B, Barda-Saad M. Matalon O, et al. Immunol Rev. 2013 Nov;256(1):10-29. doi: 10.1111/imr.12112. Immunol Rev. 2013. PMID: 24117810 Review.
Cited by
- Computational spatiotemporal analysis identifies WAVE2 and cofilin as joint regulators of costimulation-mediated T cell actin dynamics.
Roybal KT, Buck TE, Ruan X, Cho BH, Clark DJ, Ambler R, Tunbridge HM, Zhang J, Verkade P, Wülfing C, Murphy RF. Roybal KT, et al. Sci Signal. 2016 Apr 19;9(424):rs3. doi: 10.1126/scisignal.aad4149. Sci Signal. 2016. PMID: 27095595 Free PMC article. - Signal Integration during T Lymphocyte Activation and Function: Lessons from the Wiskott-Aldrich Syndrome.
Cotta-de-Almeida V, Dupré L, Guipouy D, Vasconcelos Z. Cotta-de-Almeida V, et al. Front Immunol. 2015 Feb 9;6:47. doi: 10.3389/fimmu.2015.00047. eCollection 2015. Front Immunol. 2015. PMID: 25709608 Free PMC article. Review. - Polarity of CD4+ T cells towards the antigen presenting cell is regulated by the Lck adapter TSAd.
Abrahamsen G, Sundvold-Gjerstad V, Habtamu M, Bogen B, Spurkland A. Abrahamsen G, et al. Sci Rep. 2018 Sep 6;8(1):13319. doi: 10.1038/s41598-018-31510-6. Sci Rep. 2018. PMID: 30190583 Free PMC article. - Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76.
Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, Koretzky GA. Jordan MS, et al. Immunity. 2008 Mar;28(3):359-69. doi: 10.1016/j.immuni.2008.01.010. Immunity. 2008. PMID: 18342008 Free PMC article. - Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells.
Görgün G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Görgün G, et al. J Clin Invest. 2005 Jul;115(7):1797-805. doi: 10.1172/JCI24176. Epub 2005 Jun 16. J Clin Invest. 2005. PMID: 15965501 Free PMC article.
References
- Cannon JL, Labno CM, Bosco G, Seth A, McGavin MH, Siminovitch KA, Rosen MK, Burkhardt JK. Wasp recruitment to the T cell: APC contact site occurs independently of cdc42 activation. Immunity. 2001;15:249–259. - PubMed
- Takesono A, Finkelstein LD, Schwartzberg PL. Beyond calcium: new signaling pathways for Tec family kinases. J Cell Sci. 2002;115:3039–3048. - PubMed
- Schaeffer EM, Schwartzberg PL. Tec family kinases in lymphocyte signaling and function. Curr Opin Immunol. 2000;12:282–288. - PubMed
- Snapper SB, Rosen FS. The Wiskott-Aldrich syndrome protein (WASP): roles in signaling and cytoskeletal organization. Annu Rev Immunol. 1999;17:905–929. - PubMed
- Turner M, Billadeau DD. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol. 2002;2:476–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI044835/AI/NIAID NIH HHS/United States
- R01 GM056322/GM/NIGMS NIH HHS/United States
- R01-AI44835/AI/NIAID NIH HHS/United States
- R01-GM56322/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous