Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma-/- knock-out mice - PubMed (original) (raw)
Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma-/- knock-out mice
Pritam Das et al. J Neurosci. 2003.
Abstract
Direct immunization with amyloid beta protein (Abeta) and passive transfer of anti-Abeta antibodies reduce Abeta accumulation and attenuate cognitive deficits in transgenic models of Alzheimer's disease (AD). The reduction in Abeta deposition has been proposed to involve microglial phagocytosis of Abeta immune complexes via Fc receptors (FcRs). We have examined the efficacy of Abeta immunization in amyloid precursor protein (APP) transgenic mice crossed into FcR-gamma chain knock-out mice (FcRgamma-/-). As might be expected from previous studies on macrophages, phagocytosis of Abeta immune complexes via FcR was completely impaired in microglia cells isolated from FcRgamma-/- mice. Thus, we immunized APP Tg2576 transgenic mice that were crossed in the FcRgamma-/- background with Abeta1-42 and then analyzed the effect on Abeta accumulation. In APP Tg2576 transgenic mice crossed to FcRgamma-/-, Abeta1-42 immunization significantly attenuated Abeta deposition, as assessed by both biochemical and immunohistological methods. The reduction in Abeta accumulation was equivalent to the reduction in deposition seen in Abeta1-42 immunized, age-matched, FcR-sufficient Tg2576 mice. We conclude that after Abeta immunization, the effects of anti-Abeta antibodies on Abeta deposition in APP Tg2576 transgenic mice are not dependent on FcR-mediated phagocytic events.
Figures
Figure 1.
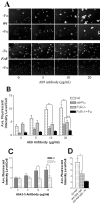
Microglia from FcRγ-/- mice exhibit defective uptake of anti-Aβ immune complexes. A, Microglia isolated from wild-type (Wt, top panels) mice and FcRγ-/- mice (bottom panels) were incubated with Cy3–Aβ in the presence of increasing concentrations of Ab9 antibody for 10 min without fucoidan (-Fu) or in the presence of 500μg/ml fucoidan (+Fu). Magnification, 40×. Data represented are from one of three independent experiments. B, Quantitation of Cy3–Aβ internalization in the presence of increasing concentrations of Ab9 and fucoidan. *p < 0.01; **p < 0.01. C, Quantitation of Cy3–Aβ internalization in the presence of increasing concentrations of Ab42–5. *p < 0.01. D, Quantitation of Cy3–Aβ internalization by wt microglia in the presence of competing IgG. Microglia were incubated with Cy3–Aβ complexed with 20 μg/ml of Ab9 antibody (Aβ + Ab9), in the presence of 100 μg of purified mouse IgG (Aβ + Ab9 + IgG), and in the presence of 500 μg/ml of fucoidan (Aβ + Ab9 + IgG) + Fu. *p < 0.01. Data represented are from one of two independent experiments.
Figure 2.
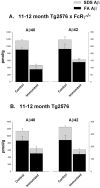
Aβ levels were significantly reduced in the Aβ1–42-immunized 11- to 12-month-old Tg2576 × FcRγ-/- mice. Mice were killed after immunization with Aβ1–42 for 3 months, and both SDS-soluble (SDS) and SDS-insoluble formic acid extractable (FA) fractions were analyzed by capture ELISA. A, Tg2576 × FcRγ-/- mice, 11–12 month old (n = 5 per group); B, wt Tg2576 mice, 11–12 month old (n = 6 per group). Statistical analyses are provided in Results.
Figure 3.
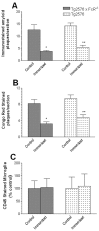
Quantitative image analysis of amyloid plaque burden in the 11- to 12-month-old Tg2576 × FcRγ-/- mice. A, Immunostained amyloid plaque burden is reduced in the 11- to 12-month-old Tg2576 × FcRγ-/- mice immunized with Aβ1–42 (*p < 0.004) and in the 11- to 12-month-old Tg2576 mice (**p < 0.005). B, Congo red-stained plaque burden is significantly reduced in the 11- to 12-month-old Tg2576 × FcRγ-/- mice immunized with Aβ1–42 (*p < 0.005) and in the 11- to 12-month-old Tg2576 mice (**p < 0.05). C, Quantitation of CD45-stained activated microglia revealed no statistically significant differences between immunized and control groups of the 11- to 12-month-old Tg2576 × FcRγ-/- and the 11- to 12-month-old Tg2576 mice.
Figure 4.
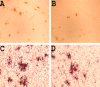
Representative pictures of immunostained Aβ plaques (stained with Ab9 antibody) in the neo cortex of 11- to 12-month-old Tg2576 × FcRγ-/- mice (A) and control group (B) immunized with Aβ1–42 (A, B, magnification 40×). Representative pictures of Congo red-stained plaques (red) decorated with microglia immunostained with anti-mouse CD45 (black) in the neo cortex of 11- to 12-month-old Tg2576 × FcRγ-/- mice (C) and control group (D) immunized with Aβ1–42 (C, D, magnification 100×).
Similar articles
- Reduced effectiveness of Abeta1-42 immunization in APP transgenic mice with significant amyloid deposition.
Das P, Murphy MP, Younkin LH, Younkin SG, Golde TE. Das P, et al. Neurobiol Aging. 2001 Sep-Oct;22(5):721-7. doi: 10.1016/s0197-4580(01)00245-7. Neurobiol Aging. 2001. PMID: 11705631 - Maternal antibodies facilitate Amyloid-β clearance by activating Fc-receptor-Syk-mediated phagocytosis.
Illouz T, Nicola R, Ben-Shushan L, Madar R, Biragyn A, Okun E. Illouz T, et al. Commun Biol. 2021 Mar 12;4(1):329. doi: 10.1038/s42003-021-01851-6. Commun Biol. 2021. PMID: 33712740 Free PMC article. - Antibody-mediated phagocytosis of the amyloid beta-peptide in microglia is differentially modulated by C1q.
Webster SD, Galvan MD, Ferran E, Garzon-Rodriguez W, Glabe CG, Tenner AJ. Webster SD, et al. J Immunol. 2001 Jun 15;166(12):7496-503. doi: 10.4049/jimmunol.166.12.7496. J Immunol. 2001. PMID: 11390503 - Novel Abeta immunogens: is shorter better?
Lemere CA, Maier M, Peng Y, Jiang L, Seabrook TJ. Lemere CA, et al. Curr Alzheimer Res. 2007 Sep;4(4):427-36. doi: 10.2174/156720507781788800. Curr Alzheimer Res. 2007. PMID: 17908047 Review. - The role of microglia in antibody-mediated clearance of amyloid-beta from the brain.
Morgan D. Morgan D. CNS Neurol Disord Drug Targets. 2009 Mar;8(1):7-15. doi: 10.2174/187152709787601821. CNS Neurol Disord Drug Targets. 2009. PMID: 19275633 Review.
Cited by
- Pittsburgh compound B and the postmortem diagnosis of Alzheimer disease.
Niedowicz DM, Beckett TL, Matveev S, Weidner AM, Baig I, Kryscio RJ, Mendiondo MS, LeVine H 3rd, Keller JN, Murphy MP. Niedowicz DM, et al. Ann Neurol. 2012 Oct;72(4):564-70. doi: 10.1002/ana.23633. Ann Neurol. 2012. PMID: 23109151 Free PMC article. - Preventive immunization of aged and juvenile non-human primates to β-amyloid.
Kofler J, Lopresti B, Janssen C, Trichel AM, Masliah E, Finn OJ, Salter RD, Murdoch GH, Mathis CA, Wiley CA. Kofler J, et al. J Neuroinflammation. 2012 May 3;9:84. doi: 10.1186/1742-2094-9-84. J Neuroinflammation. 2012. PMID: 22554253 Free PMC article. - Amyloid-beta immunotherapy for Alzheimer's disease.
Fu HJ, Liu B, Frost JL, Lemere CA. Fu HJ, et al. CNS Neurol Disord Drug Targets. 2010 Apr;9(2):197-206. doi: 10.2174/187152710791012017. CNS Neurol Disord Drug Targets. 2010. PMID: 20205640 Free PMC article. Review. - Novel Abeta peptide immunogens modulate plaque pathology and inflammation in a murine model of Alzheimer's disease.
Zhou J, Fonseca MI, Kayed R, Hernandez I, Webster SD, Yazan O, Cribbs DH, Glabe CG, Tenner AJ. Zhou J, et al. J Neuroinflammation. 2005 Dec 7;2:28. doi: 10.1186/1742-2094-2-28. J Neuroinflammation. 2005. PMID: 16332263 Free PMC article. - BACE1 and BACE2 enzymatic activities in Alzheimer's disease.
Ahmed RR, Holler CJ, Webb RL, Li F, Beckett TL, Murphy MP. Ahmed RR, et al. J Neurochem. 2010 Feb;112(4):1045-53. doi: 10.1111/j.1471-4159.2009.06528.x. Epub 2009 Dec 4. J Neurochem. 2010. PMID: 19968762 Free PMC article.
References
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT ( 2001) Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med 7: 369-372. - PubMed
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T ( 2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916-919. - PubMed
- Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T ( 2003) Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc Natl Acad Sci USA 100: 2023-2028. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials