Human immunodeficiency virus-1 Nef expression induces intracellular accumulation of multivesicular bodies and major histocompatibility complex class II complexes: potential role of phosphatidylinositol 3-kinase - PubMed (original) (raw)
Human immunodeficiency virus-1 Nef expression induces intracellular accumulation of multivesicular bodies and major histocompatibility complex class II complexes: potential role of phosphatidylinositol 3-kinase
Pamela Stumptner-Cuvelette et al. Mol Biol Cell. 2003 Dec.
Abstract
Nef alters the cell surface expression of several immunoreceptors, which may contribute to viral escape. We show that Nef modifies major histocompatibility complex class II (MHC II) intracellular trafficking and thereby its function. In the presence of Nef, mature, peptide-loaded MHC II were down-modulated at the cell surface and accumulated intracellularly, whereas immature (invariant [Ii] chain-associated) MHC II expression at the plasma membrane was increased. Antibody internalization experiments and subcellular fractionation analyses showed that immature MHC II were internalized from the plasma membrane but had limited access to lysosomes, explaining the reduced Ii chain degradation. Immunoelectron microscopy revealed that Nef expression induced a marked accumulation of multivesicular bodies (MVBs) containing Nef, MHC II, and high amounts of Ii chain. The Nef-induced up-regulation of surface Ii chain was inhibited by LY294002 exposure, indicating the involvement of a phosphatidylinositol 3-kinase, whose products play a key role in MVB biogenesis. Together, our results indicate that Nef induces an increase of the number of MVBs where MHC II complexes accumulate. Given that human immunodeficiency virus recruits the MVB machinery for its assembly process, our data raise the possibility that Nef is involved in viral assembly through its effect on MVBs.
Figures
Figure 1.
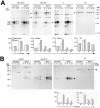
Nef reduces Ii chain degradation. HeLa-CIITA cells transfected with Nef-FT or Nef-mock were pulse-labeled with 35S-met and -cys for 15 min and chased for 0, 4, 8, or 22 h. At the end of the chase cells were surface-biotinylated before lysis and immunoprecipitation with PIN1 (anti-Ii chain), 1B5 (anti-DRα), or 66Ig10 (anti-TfR) mAbs. (A) Immunoprecipitates eluted at 95°C or in the case of the DRα (RT) panel at room temperature, were analyzed by SDS-PAGE. Quantification by phosphorimage analyses of the indicated chains or complexes (see arrowheads) are represented as histograms below the gels (arbitrary units). Ii trimers designate αβIi complexes. (B) The biotinylated fraction of the immunoprecipitated molecules analyzed in A were reprecipitated with streptavidin-agarose beads and analyzed by SDS-PAGE. Therefore the streptavidin-precipitated material represents molecules expressed at the cell surface.
Figure 2.
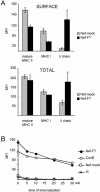
Nef induces intracellular accumulation of mature MHC II. (A) HeLa-CIITA cells transfected with Nef-FT or Nef-mock were stained in parallel for expression of mature MHC II, MHC I, and Ii chain either at the cell surface of intact cells or in total in permeabilized cells by using L243, W6/32, and LN-2 mAbs, respectively. MFI values of GFP+ gated cells were determined for the indicated markers in FL-2. The background MFI value (obtained with a control mAb) was subtracted from these MFI values, which were then represented for the indicated molecules. (B) HeLa-CIITA cells transfected 24 h earlier with either Nef-FT or Nef-mock and pEGFP, or nontransfected cells previously exposed to 20 nM ConB, were collected and incubated with an anti-Ii antibody for 30 min on ice. After washes, cells were chased at 37°C for the indicated period of time, at the end of which eventual surface antibodies were revealed with secondary antibodies labeled with PE. Analysis by FACS of PE-fluorescence of GFP+ cells was performed (except in the case of ConB-treated cells, where all cells were analyzed). MFIs are plotted as a function of time of internalization.
Figure 3.
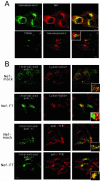
Nef expression impedes access of internalized immature MHC II complexes to lysosomal compartments. Confocal microscopy of HeLa-CIITA cells transfected with Nef-mock or Nef-FT. Confocal sections of the indicated stainings are presented together with their overlays that contain an insert representing a higher magnification of the same field. Bars, 10 μm. (A) Cells were incubated with either rabbit (top row) or mouse (bottom row) anti-Ii chain antibodies for 1 h at 19.5°C and then chased for 90 min at 37°C. After washes and fixation, remaining anti-Ii antibodies were revealed with appropriate FITC-labeled secondary antibodies. Cells were costained with either the anti-Nef mAb FMATG-Cy3 or with a sheep anti-TGN46 immunosera revealed by Cy3-labeled secondary antibodies. Nef and anti-Ii chain stainings clearly overlap in the top row. Although very close, TGN46 and Ii chain specific antibodies do not colocalize in Nef-expressing cells, note the magnification in the inset. (B) Two upper rows: cells were incubated for 1 h at 19.5°C with the anti-Ii mAb LN-2, washed, and chased for 90 min at 37°C in the presence of LysoTracker, which labels acidic compartments in red. Remaining LN-2 was revealed with FITC-labeled secondary antibodies. Note the abundant colocalization of both stainings in control cells (Nef-mock). Nef-transfected cells can be easily identified by the increased amount of internalized anti-Ii antibody, which reaches acidic compartments to a much lesser extent than in control cells. Two lower rows: transfection and internalization were carried out as described above except for the use of rabbit anti-Ii immune serum revealed by FITC-labeled anti-rabbit antibodies. Cells were costained with an anti-TfR mAb revealed by Cy3-labeled secondary antibodies. Anti-Ii antibodies codistribute partially with TfR in cells transfected with Nef-FT and to a much lower extent in control cells.
Figure 4.
Specificity of Nef effect on transport to lysosomal compartments. HeLa-CIITA cells infected 24 h earlier with Ad-HA or with Ad-Nef-IRES-HA were disrupted by a ball-bearing homogenizer and fractionated on a Percoll density gradient. Aliquots of each fraction were analyzed by SDS-PAGE followed by immunoblotting for expression of HLA-DMβ (with HLA-DM-K8 mAb), Lamp-1 (with anti-Lamp-1 mAb), Invariant chain (with rabbit anti-Ii immunoserum), HLA-DRα (with 1B5 mAb) and Nef (FMATG 20 mAb). In the top left corner, Western blots of Ii, HLA-DRα, and Nef are presented. In parallel, β-hexosaminidase activity was measured in each fraction. Histogram quantifications of the indicated proteins are presented for both cell populations. The heavy fractions of both cell types contained similar levels of β-hexosaminidase activity and of HLA-DMβ and Lamp-1 proteins. Compared with control cells, Nef-expressing cells contained higher amounts of Ii chain and HLA-DRα chains in their light fractions, whereas lesser amounts of both chains were detected in their heavy fractions.
Figure 5.
Immunoelectron microscopy analysis of Nef-expressing cells. Ultrathin cryosections of HeLa-CIITA cells transfected with Nef-FT were double immunogold-labeled with the anti-Nef mAb MATG (protein A gold [PAG] 15) and the anti-DR polyclonal antibody (PAG 10). Bar, 200 nm. Nef is associated with numerous membrane vesicles and tubulovesicular elements at the TGN. The Nef protein is also associated with the cytosolic side of the limiting membrane of MVB (top inset, arrows) and closely apposed tubulovesicular structures (bottom inset) that are often coated (see arrow). Bar (insets), 100 nm.
Figure 6.
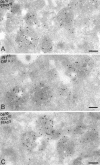
Nef-expressing cells accumulate MVBs. (A) Ultrathin cryosections of HeLa-CIITA cells transfected with Nef-FT were single immuno-gold labeled with antibodies directed against HLA-DR (PAG 10). This micrograph shows two typical MHC II+ MVBs observed in Nef-expressing cells with tightly packed internal vesicles. (B) Lysosomes in HeLa-CIITA cells transfected with Nef-FT display multilaminar membranes and contain Lamp-1 (PAG 15) and MHC II (PAG 10). (C) Ultrathin cryosection of mock-transfected HeLa-CIITA cells were double immunogold labeled for Lamp-1 (PAG 15) and MHC II (PAG 10). Note the MVB immunogold labeled only for MHC II (MVB) and lysosomes containing both MHC II and Lamp-1. Bar, 100 nm.
Figure 8.
Accumulated MVBs in Nef-expressing cells contain CD63, MHC II, and Ii chain. Ultrathin cryosections of HeLa-CIITA cells transfected with Nef-FT were double labeled with anti-HLA-DR antibodies (protein A gold [PAG] 10) and (A) an anti-CD63 mAb (PAG 15), (B) anticytoplasmic tail of the Ii chain (PAG 15) and (C) antilumenal side of the Ii chain antibodies (PAG 15). In addition, cryosections on C were prepared from cells transfected with Nef-FT and incubated with BSA coupled to 5-nm colloidal gold for 90 min at 37°C and then fixed. Numerous multivesicular compartments accumulate in Nef-expressing cells that contain MHC II, CD63, and intact Ii chain and were accessible to internalized BSA-gold. Bar, 200 nm.
Figure 7.
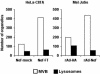
Nef-expressing cells accumulate MVBs. HeLa-CIITA cells were transfected with Nef-mock or Nef-FT. Mel JuSo cells were infected with recombinant adenovirus encoding either Nef and HA (here noted rAd-Nef) or HA (rAd-HA) (see MATERIALS AND METHODS). The numbers of MVBs and lysosomes in each cell type were counted directly under the electron microscope on ultrathin cryosections immunogold labeled for Nef.
Figure 9.
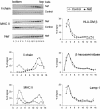
PI 3-kinase activity is required for the Nef-induced up-regulation of Ii chain at the cell surface. HeLa-CIITA cells were transfected with either Nef-FT or Nef-mock and pEGFP. (A) Cells were recovered 24 h posttransfection, stained, and analyzed by FACS. For the last 15 h, cells were either treated with 50 μM LY294002 or with its solvent, DMSO. For each cell population, MFI values obtained for the indicated surface marker in GFP+ cells are represented. Data are representative of four independent experiments. (B) Localization of Ii chain by immunofluorescence microscopy. Twenty-four hours after transfection, cells were fixed, permeabilized, and stained with rabbit anti-Ii chain polyclonal antibody revealed by Cy-3–labeled secondary antibodies. For the last 3 h, cells were either treated with 50 μM LY294002 or with its solvent, DMSO. Only Cy-3 staining of transfected cells are shown (transfected cells were identified by GFP expression). Bar, 10 μM.
Similar articles
- HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI 3-kinase-dependent pathway.
Swann SA, Williams M, Story CM, Bobbitt KR, Fleis R, Collins KL. Swann SA, et al. Virology. 2001 Apr 10;282(2):267-77. doi: 10.1006/viro.2000.0816. Virology. 2001. PMID: 11289809 - Two Functional Variants of AP-1 Complexes Composed of either γ2 or γ1 Subunits Are Independently Required for Major Histocompatibility Complex Class I Downregulation by HIV-1 Nef.
Tavares LA, de Carvalho JV, Costa CS, Silveira RM, de Carvalho AN, Donadi EA, daSilva LLP. Tavares LA, et al. J Virol. 2020 Mar 17;94(7):e02039-19. doi: 10.1128/JVI.02039-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31915283 Free PMC article. - The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes.
Greenberg ME, Iafrate AJ, Skowronski J. Greenberg ME, et al. EMBO J. 1998 May 15;17(10):2777-89. doi: 10.1093/emboj/17.10.2777. EMBO J. 1998. PMID: 9582271 Free PMC article. - HIV-1 Nef: Taking Control of Protein Trafficking.
Pereira EA, daSilva LL. Pereira EA, et al. Traffic. 2016 Sep;17(9):976-96. doi: 10.1111/tra.12412. Epub 2016 Jun 3. Traffic. 2016. PMID: 27161574 Review. - HIV-1 Nef: a master manipulator of the membrane trafficking machinery mediating immune evasion.
Pawlak EN, Dikeakos JD. Pawlak EN, et al. Biochim Biophys Acta. 2015 Apr;1850(4):733-41. doi: 10.1016/j.bbagen.2015.01.003. Epub 2015 Jan 10. Biochim Biophys Acta. 2015. PMID: 25585010 Review.
Cited by
- Human immunodeficiency virus type 1 nef expression prevents AP-2-mediated internalization of the major histocompatibility complex class II-associated invariant chain.
Toussaint H, Gobert FX, Schindler M, Banning C, Kozik P, Jouve M, Kirchhoff F, Benaroch P. Toussaint H, et al. J Virol. 2008 Sep;82(17):8373-82. doi: 10.1128/JVI.00670-08. Epub 2008 Jul 2. J Virol. 2008. PMID: 18596106 Free PMC article. - Slow Release of HIV-1 Protein Nef from Vesicle-like Structures Is Inhibited by Cytosolic Calcium Elevation in Single Human Microglia.
Stenovec M, Lasič E, Dominkuš PP, Bobnar ST, Zorec R, Lenassi M, Kreft M. Stenovec M, et al. Mol Neurobiol. 2019 Jan;56(1):102-118. doi: 10.1007/s12035-018-1072-2. Epub 2018 Apr 21. Mol Neurobiol. 2019. PMID: 29679260 - The HIV-1 Nef protein binds argonaute-2 and functions as a viral suppressor of RNA interference.
Aqil M, Naqvi AR, Bano AS, Jameel S. Aqil M, et al. PLoS One. 2013 Sep 4;8(9):e74472. doi: 10.1371/journal.pone.0074472. eCollection 2013. PLoS One. 2013. PMID: 24023945 Free PMC article. - Autophagy and HIV.
Dinkins C, Arko-Mensah J, Deretic V. Dinkins C, et al. Semin Cell Dev Biol. 2010 Sep;21(7):712-8. doi: 10.1016/j.semcdb.2010.04.004. Epub 2010 Apr 18. Semin Cell Dev Biol. 2010. PMID: 20403451 Free PMC article. Review. - Exosome-mediated regulation of inflammatory pathway during respiratory viral disease.
Gheitasi H, Sabbaghian M, Shekarchi AA, Mirmazhary AA, Poortahmasebi V. Gheitasi H, et al. Virol J. 2024 Jan 25;21(1):30. doi: 10.1186/s12985-024-02297-y. Virol J. 2024. PMID: 38273382 Free PMC article. Review.
References
- Aiken, C., Konner, J., Landau, N.R., Lenburg, M.E., and Trono, D. (1994). Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76, 853–864. - PubMed
- Bachelerie, F., Alcami, J., Hazan, U., Israel, N., Goud, B., Arenzana-Seisdedos, F., and Virelizier, J.L. (1990). Constitutive expression of human immunodeficiency virus (HIV) nef protein in human astrocytes does not influence basal or induced HIV long terminal repeat activity. J. Virol. 64, 3059–3062. - PMC - PubMed
- Blagoveshchenskaya, A.D., Thomas, L., Feliciangeli, S.F., Hung, C.H., and Thomas, G. (2002). HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111, 853–866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials