A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection - PubMed (original) (raw)
A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection
Ann E Jerse et al. Infect Immun. 2003 Oct.
Abstract
Active efflux of antimicrobial substances is likely to be an important bacterial defense against inhibitory host factors inherent to different body sites. Two well-characterized multidrug resistance efflux systems (MtrCDE and FarAB-MtrE) exist in Neisseria gonorrhoeae, a bacterial pathogen of the human genital mucosae. In vitro studies suggest that the MtrCDE and FarAB-MtrE efflux systems protect the gonococcus from hydrophobic antimicrobial substances that are likely to be present on mucosal surfaces. Here we report that a functional MtrCDE efflux system, but not a functional FarAB-MtrE system, enhances experimental gonococcal genital tract infection in female mice. Specifically, the recovery of mtrD and mtrE mutants, but not a farB mutant, from mice inoculated with mutant or wild-type gonococci was reduced compared with that of the wild-type strain. Competitive-infection experiments confirmed the survival disadvantage of MtrCDE-deficient gonococci. This report is the first direct evidence that a multidrug resistance efflux system enhances survival of a bacterial pathogen in the genital tract. Additionally, experiments using ovariectomized mice showed that MtrCDE-deficient gonococci were more rapidly cleared from mice that were capable of secreting gonadal hormones. MtrCDE-deficient gonococci were more sensitive to nonphysiological concentrations of progesterone in vitro than were wild-type or FarAB-MtrE-deficient gonococci. These results suggest that progesterone may play an inhibitory role in vivo. However, hormonally regulated factors rather than progesterone itself may be responsible for the more rapid clearance of mtr-deficient gonococci from intact mice.
Figures
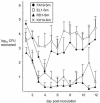
FIG. 1.
Recovery of wild-type and efflux pump-deficient N. gonorrhoeae from estradiol-treated mice. Results are expressed as the average number of CFU (expressed as log10) recovered from vaginal swab suspensions over a 12-day period. P = 0.012 and 0.024 for KH14-Sm and RD1-Sm, respectively, compared with the wild-type strain. The experiment was performed twice, and the results were similar.
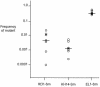
FIG. 2.
Recovery of efflux pump-deficient gonococci relative to that of the wild-type strain in competitive infections. The frequency of the mutant in the total population of gonococci recovered for each mouse in each experimental group on day 4 postinoculation is shown on the y axis. The frequency of mutant CFU within the inocula ranged from 0.35 to 0.59. The medians are represented by horizontal bars. P = 0.003 for RD1-Sm and KH14-Sm when compared with the recovery of EL1-Sm (as determined by the Mann-Whitney test). The experiment was performed three times using four to seven mice per group, and the results were similar.
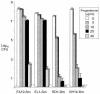
FIG. 3.
Plating efficiency of efflux pump-deficient N. gonorrhoeae on agar containing progesterone. GC agar contained 0, 5, 10, 20, and 40 μg of progesterone/ml. The experiment was performed twice, and the results were similar.
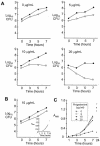
FIG. 4.
Inhibition of Mtr-deficient N. gonorrhoeae by progesterone in mixed broth cultures. Mutants RD1-Sm and EL1-Sm were cocultured with wild-type FA19-Sm in GC broth containing 0 to 20 μg of progesterone/ml. (A) Recovery of bacteria on solid agar without antibiotic selection (closed circles, total CFU) versus that on agar containing kanamycin (open circles, mutant CFU) from FA19-Sm-RD1-Sm mixed cultures containing progesterone at the concentrations indicated on each graph. (B) Recovery of mutant EL1-Sm (open circles) when cocultured with FA19-Sm in the presence of 10 μg of progesterone/ml (closed circles, total CFU) and the change in absorbency of this culture over time (inset). (C) Total bacterial growth within FA19-Sm-RD1-Sm mixed cultures in the presence of increasing concentrations of progesterone as measured by change in absorbency at 600 nm. The experiments were performed twice, and the results were similar.
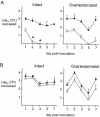
FIG. 5.
Recovery of efflux pump-deficient gonococci relative to that of the wild-type strain in competitive-infection experiments using intact versus ovariectomized mice. Results are expressed as the total number of CFU (closed circles) and the number of Kmr CFU (mutant) (open circles) recovered from mice inoculated with mixed suspensions containing a 1:1 ratio of wild-type FA19 to mutant RD1-Sm (A) or mutant EL1-Sm (B). Asterisks indicate time points at which significant differences in the recovery of RD1-Sm gonococci were detected in intact versus ovariectomized mice (P = 0.012 and 0.013 on days 2 and 3, respectively). The experiment was performed twice with six to nine mice per group, and the results were similar.
Similar articles
- Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae.
Warner DM, Folster JP, Shafer WM, Jerse AE. Warner DM, et al. J Infect Dis. 2007 Dec 15;196(12):1804-12. doi: 10.1086/522964. J Infect Dis. 2007. PMID: 18190261 - Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE Efflux pump system confer different levels of antimicrobial resistance and in vivo fitness.
Warner DM, Shafer WM, Jerse AE. Warner DM, et al. Mol Microbiol. 2008 Oct;70(2):462-78. doi: 10.1111/j.1365-2958.2008.06424.x. Epub 2008 Aug 27. Mol Microbiol. 2008. PMID: 18761689 Free PMC article. - The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids.
Lee EH, Shafer WM. Lee EH, et al. Mol Microbiol. 1999 Aug;33(4):839-45. doi: 10.1046/j.1365-2958.1999.01530.x. Mol Microbiol. 1999. PMID: 10447892 - Transcriptional regulation of the mtrCDE efflux pump operon: importance for Neisseria gonorrhoeae antimicrobial resistance.
Ayala JC, Balthazar JT, Shafer WM. Ayala JC, et al. Microbiology (Reading). 2022 Aug;168(8):001231. doi: 10.1099/mic.0.001231. Microbiology (Reading). 2022. PMID: 35916832 Free PMC article. Review. - Genetic organization and regulation of antimicrobial efflux systems possessed by Neisseria gonorrhoeae and Neisseria meningitidis.
Shafer WM, Veal WL, Lee EH, Zarantonelli L, Balthazar JT, Rouquette C. Shafer WM, et al. J Mol Microbiol Biotechnol. 2001 Apr;3(2):219-24. J Mol Microbiol Biotechnol. 2001. PMID: 11321577 Review.
Cited by
- Role of sex steroid hormones in bacterial-host interactions.
García-Gómez E, González-Pedrajo B, Camacho-Arroyo I. García-Gómez E, et al. Biomed Res Int. 2013;2013:928290. doi: 10.1155/2013/928290. Epub 2012 Dec 24. Biomed Res Int. 2013. PMID: 23509808 Free PMC article. Review. - Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future.
Unemo M, Shafer WM. Unemo M, et al. Ann N Y Acad Sci. 2011 Aug;1230:E19-28. doi: 10.1111/j.1749-6632.2011.06215.x. Ann N Y Acad Sci. 2011. PMID: 22239555 Free PMC article. Review. - Could Dampening Expression of the Neisseria gonorrhoeae _mtrCDE_-Encoded Efflux Pump Be a Strategy To Preserve Currently or Resurrect Formerly Used Antibiotics To Treat Gonorrhea?
Chen S, Connolly KL, Rouquette-Loughlin C, D'Andrea A, Jerse AE, Shafer WM. Chen S, et al. mBio. 2019 Aug 13;10(4):e01576-19. doi: 10.1128/mBio.01576-19. mBio. 2019. PMID: 31409679 Free PMC article. - PubMLST for Antigen Allele Mining to Inform Development of Gonorrhea Protein-Based Vaccines.
Baarda BI, Zielke RA, Nicholas RA, Sikora AE. Baarda BI, et al. Front Microbiol. 2018 Dec 7;9:2971. doi: 10.3389/fmicb.2018.02971. eCollection 2018. Front Microbiol. 2018. PMID: 30581422 Free PMC article. - Complete Genome Sequencing of Leptospira interrogans Isolates from Malaysia Reveals Massive Genome Rearrangement but High Conservation of Virulence-Associated Genes.
Ramli SR, Bunk B, Spröer C, Geffers R, Jarek M, Bhuju S, Goris M, Mustakim S, Pessler F. Ramli SR, et al. Pathogens. 2021 Sep 15;10(9):1198. doi: 10.3390/pathogens10091198. Pathogens. 2021. PMID: 34578230 Free PMC article.
References
- Brabin, L. 2002. Interactions of the female hormonal environment, susceptibility to viral infection, and disease progression. AIDS Patient Care STDs 16:211-221. - PubMed
- Braude, A. I., L. B. Corbeil, S. Levine, J. Ito, and J. A. McCutchan. 1978. Possible influence of cyclic menstrual changes on resistance to the gonococcus, p. 328-337. In G. F. Brooks, E. C. Gotschlich, K. K. Holmes, W. D. Sawyer, and F. E. Young (ed.), Immunobiology of Neisseria gonorrhoeae. American Society for Microbiology, Washington, D.C.
- Chattoraj, S. C. 1976. Endocrine function, p. 699-823. In N. W. Tietz (ed.), Fundamentals of clinical chemistry. W. B. Saunders Company, Philadelphia, Pa.
- Critchley, H. O. D., R. W. Kelly, R. M. Brenner, and D. T. Baird. 2001. The endocrinology of menstruation—a role for the immune system. Clin. Endocrinol. 55:701-710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources