EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells - PubMed (original) (raw)
. 2003 Sep 30;100(20):11606-11.
doi: 10.1073/pnas.1933744100. Epub 2003 Sep 19.
Qi Cao, Sooryanarayana Varambally, Ronglai Shen, Ichiro Ota, Scott A Tomlins, Debashis Ghosh, Richard G A B Sewalt, Arie P Otte, Daniel F Hayes, Michael S Sabel, Donna Livant, Stephen J Weiss, Mark A Rubin, Arul M Chinnaiyan
Affiliations
- PMID: 14500907
- PMCID: PMC208805
- DOI: 10.1073/pnas.1933744100
EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells
Celina G Kleer et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The Polycomb Group Protein EZH2 is a transcriptional repressor involved in controlling cellular memory and has been linked to aggressive prostate cancer. Here we investigate the functional role of EZH2 in cancer cell invasion and breast cancer progression. EZH2 transcript and protein were consistently elevated in invasive breast carcinoma compared with normal breast epithelia. Tissue microarray analysis, which included 917 samples from 280 patients, demonstrated that EZH2 protein levels were strongly associated with breast cancer aggressiveness. Overexpression of EZH2 in immortalized human mammary epithelial cell lines promotes anchorage-independent growth and cell invasion. EZH2-mediated cell invasion required an intact SET domain and histone deacetylase activity. This study provides compelling evidence for a functional link between dysregulated cellular memory, transcriptional repression, and neoplastic transformation.
Figures
Fig. 1.
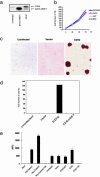
EZH2 mRNA transcript and protein levels are elevated in breast cancer. (a) Quantitative SYBR green RT-PCR of EZH2 transcript in laser-capture microdissected normal and breast cancer epithelia. Each sample was performed in duplicate, and a ratio was calculated relative to the housekeeping gene hydroxymethylbilane synthase (HMBS). (b) Immunoblot analysis of EZH2 and EED in breast tissue extracts. Metastatic (Met) prostate cancer was used as a positive control. β-Tubulin was included as a loading control. (c) Representative breast tissue sections stained with an antibody to EZH2. (Left) Normal breast epithelia (open triangle) and adjacent intravascular breast cancer emboli (filled triangle). (Center) An invasive breast cancer expressing high levels of EZH2. (Right) An invasive breast cancer expressing low levels of EZH2. (d) Tissue microarray analysis of EZH2 expression in breast cancer progression. Tumor specimens were stratified into high EZH2 expressors (filled bars, scored 3 or 4) and low EZH2 expressors (open bars, scored 1 or 2). The y axis represents the percentage of patients in each category.
Fig. 2.
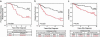
High EZH2 levels are associated with aggressive breast cancer. (a) Kaplan–Meier analysis of metastasis-free survival according to EZH2 mRNA transcript levels as measured using DNA microarrays by van't Veer et al. (30). Kaplan–Meier analysis of disease-specific (b) and overall (c) survival according to EZH2 protein levels as assessed by immunohistochemical analysis. Patients grouped on the basis of high (+) or low (–) EZH2 expression levels. P values were calculated by using the log-rank test.
Fig. 3.
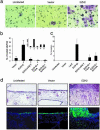
Anchorage-independent growth mediated by EZH2. (a) Immunoblot analysis of breast cell line H16N2 infected with adenovirus encoding EZH2 or EZH2 Δ SET mutant. (b) Ectopic overexpression of EZH2 does not significantly enhance growth of breast epithelial cells in culture. H16N2 cells were infected with EZH2 adenovirus and controls, and cells were counted at indicated time points. LacZ adenovirus and vector adenovirus were used as controls. (c) EZH2 expression enhances anchorage-independent growth in vitro. H16N2 cells were infected with EZH2, EZH2 Δ SET, or vector adenoviruses. Anchorage-independent growth was determined by assaying colony formation in soft agar as described in Methods. After 25 days, the plates were stained and photographed. (d) Quantitation of soft agar colonies from experiments described in c. Colonies from three wells were quantitated for each condition. (e) EZH2 induces HDAC activity in breast epithelial cells. HDAC activity was measured in extracts from H16N2 cells infected with indicated viruses ± treatment with TSA (1.0 μM). As indicated by the manufacturer (Biomol), nuclear extracts from HeLa cells were used as positive controls. Extract 2 had 2-fold more HDAC activity than Extract 1. AFU, arbitrary fluorescence units.
Fig. 4.
EZH2 orchestrates cell invasion both in vitro and in vivo.(a) A reconstituted basement membrane invasion chamber assay (Chemicon) was used to assess breast epithelial cell lines infected with EZH2 and control adenoviruses. Representative fields of invaded and stained cells are shown. (b) The numbers of invaded cells were counted in six fields, and the mean values were determined. Quantitation by colorimetry (absorbance at 560 nm) is shown in Inset.(c) EZH2-mediated invasion of SU-ECM. H16N2 cells were infected with EZH2, EZH2 Δ SET, or control adenoviruses. (d) EZH2 overexpression mediates invasion of breast epithelial cells in a CAM assay. (Upper) CAM tissues stained with hematoxylin/eosin. Arrows indicate the cells that have invaded the CAM. Because cells were labeled with Fluoresbrite carboxylated polystyrene nanospheres, they could also be visualized by fluorescence (Lower).
Similar articles
- Polycomb group protein enhancer of zeste 2 is an oncogene that promotes the neoplastic transformation of a benign prostatic epithelial cell line.
Karanikolas BD, Figueiredo ML, Wu L. Karanikolas BD, et al. Mol Cancer Res. 2009 Sep;7(9):1456-65. doi: 10.1158/1541-7786.MCR-09-0121. Epub 2009 Sep 1. Mol Cancer Res. 2009. PMID: 19723877 Free PMC article. - Expression levels of the EZH2 polycomb transcriptional repressor correlate with aggressiveness and invasive potential of bladder carcinomas.
Weikert S, Christoph F, Köllermann J, Müller M, Schrader M, Miller K, Krause H. Weikert S, et al. Int J Mol Med. 2005 Aug;16(2):349-53. Int J Mol Med. 2005. PMID: 16012774 - Enhancer of Zeste 2 as a marker of preneoplastic progression in the breast.
Ding L, Kleer CG. Ding L, et al. Cancer Res. 2006 Oct 1;66(19):9352-5. doi: 10.1158/0008-5472.CAN-06-2384. Cancer Res. 2006. PMID: 17018586 - Importance of Ezh2 polycomb protein in tumorigenesis process interfering with the pathway of growth suppressive key elements.
Tonini T, D'Andrilli G, Fucito A, Gaspa L, Bagella L. Tonini T, et al. J Cell Physiol. 2008 Feb;214(2):295-300. doi: 10.1002/jcp.21241. J Cell Physiol. 2008. PMID: 17786943 Review. - The Polycomb group protein Enhancer of Zeste 2: its links to DNA repair and breast cancer.
Zeidler M, Kleer CG. Zeidler M, et al. J Mol Histol. 2006 Sep;37(5-7):219-23. doi: 10.1007/s10735-006-9042-9. Epub 2006 Jul 20. J Mol Histol. 2006. PMID: 16855786 Review.
Cited by
- HOTAIR is a therapeutic target in glioblastoma.
Zhou X, Ren Y, Zhang J, Zhang C, Zhang K, Han L, Kong L, Wei J, Chen L, Yang J, Wang Q, Zhang J, Yang Y, Jiang T, Li M, Kang C. Zhou X, et al. Oncotarget. 2015 Apr 10;6(10):8353-65. doi: 10.18632/oncotarget.3229. Oncotarget. 2015. PMID: 25823657 Free PMC article. - BRCA1 is a negative modulator of the PRC2 complex.
Wang L, Zeng X, Chen S, Ding L, Zhong J, Zhao JC, Wang L, Sarver A, Koller A, Zhi J, Ma Y, Yu J, Chen J, Huang H. Wang L, et al. EMBO J. 2013 May 29;32(11):1584-97. doi: 10.1038/emboj.2013.95. Epub 2013 Apr 26. EMBO J. 2013. PMID: 23624935 Free PMC article. - Evaluation of histone 3 lysine 27 trimethylation (H3K27me3) and enhancer of Zest 2 (EZH2) in pediatric glial and glioneuronal tumors shows decreased H3K27me3 in H3F3A K27M mutant glioblastomas.
Venneti S, Garimella MT, Sullivan LM, Martinez D, Huse JT, Heguy A, Santi M, Thompson CB, Judkins AR. Venneti S, et al. Brain Pathol. 2013 Sep;23(5):558-64. doi: 10.1111/bpa.12042. Epub 2013 Mar 6. Brain Pathol. 2013. PMID: 23414300 Free PMC article. - Genomic and Epigenomic Alterations in Cancer.
Chakravarthi BV, Nepal S, Varambally S. Chakravarthi BV, et al. Am J Pathol. 2016 Jul;186(7):1724-35. doi: 10.1016/j.ajpath.2016.02.023. Am J Pathol. 2016. PMID: 27338107 Free PMC article. Review. - The Role of EZH2 Inhibitor, GSK-126, in Seizure Susceptibility.
Wang Z, Su Y, Zhuang D, Lan T. Wang Z, et al. J Mol Neurosci. 2021 Mar;71(3):556-564. doi: 10.1007/s12031-020-01677-7. Epub 2020 Aug 8. J Mol Neurosci. 2021. PMID: 32772228
References
- Jemal, A., Murray, T., Samuels, A., Ghafoor, A., Ward, E. & Thun, M. J. (2003) CA Cancer J. Clin. 53, 5–26. - PubMed
- Ellis, M., Hayes, D. & Lippman, M. (2000) in Diseases of the Breast, eds. Harris, J., Lippman, M. E. & Morrow, M. (Lippincott–Raven, Philadelpha), pp. 749–798.
- Hayes, D. F., Isaacs, C. & Stearns, V. (2001) J. Mammary Gland Biol. Neoplasia 6, 375–392. - PubMed
- Hayes, D. F., Trock, B. & Harris, A. L. (1998) Breast Cancer Res. Treat. 52, 305–319. - PubMed
- Honig, S. (1996) in Diseases of the Breast, eds. Harris, J. L. M., Morrow, M. & Hellman, S. (Lippincott–Raven, Philadelphia), pp. 461–485.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical