Human neuronal stargazin-like proteins, gamma2, gamma3 and gamma4; an investigation of their specific localization in human brain and their influence on CaV2.1 voltage-dependent calcium channels expressed in Xenopus oocytes - PubMed (original) (raw)
Human neuronal stargazin-like proteins, gamma2, gamma3 and gamma4; an investigation of their specific localization in human brain and their influence on CaV2.1 voltage-dependent calcium channels expressed in Xenopus oocytes
Fraser J Moss et al. BMC Neurosci. 2003.
Abstract
Background: Stargazin (gamma2) and the closely related gamma3, and gamma4 transmembrane proteins are part of a family of proteins that may act as both neuronal voltage-dependent calcium channel (VDCC) gamma subunits and transmembrane alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproponinc (AMPA) receptor regulatory proteins (TARPs). In this investigation, we examined the distribution patterns of the stargazin-like proteins gamma2, gamma3, and gamma4 in the human central nervous system (CNS). In addition, we investigated whether human gamma2 or gamma4 could modulate the electrophysiological properties of a neuronal VDCC complex transiently expressed in Xenopus oocytes.
Results: The mRNA encoding human gamma2 is highly expressed in cerebellum, cerebral cortex, hippocampus and thalamus, whereas gamma3 is abundant in cerebral cortex and amygdala and gamma4 in the basal ganglia. Immunohistochemical analysis of the cerebellum determined that both gamma2 and gamma4 are present in the molecular layer, particularly in Purkinje cell bodies and dendrites, but have an inverse expression pattern to one another in the dentate cerebellar nucleus. They are also detected in the interneurons of the granule cell layer though only gamma2 is clearly detected in granule cells. The hippocampus stains for gamma2 and gamma4 throughout the layers of the every CA region and the dentate gyrus, whilst gamma3 appears to be localized particularly to the pyramidal and granule cell bodies. When co-expressed in Xenopus oocytes with a CaV2.1/beta4 VDCC complex, either in the absence or presence of an alpha2delta2 subunit, neither gamma2 nor gamma4 significantly modulated the VDCC peak current amplitude, voltage-dependence of activation or voltage-dependence of steady-state inactivation.
Conclusion: The human gamma2, gamma3 and gamma4 stargazin-like proteins are detected only in the CNS and display differential distributions among brain regions and several cell types in found in the cerebellum and hippocampus. These distribution patterns closely resemble those reported by other laboratories for the rodent orthologues of each protein. Whilst the fact that neither gamma2 nor gamma4 modulated the properties of a VDCC complex with which they could associate in vivo in Purkinje cells adds weight to the hypothesis that the principal role of these proteins is not as auxiliary subunits of VDCCs, it does not exclude the possibility that they play another role in VDCC function.
Figures
Figure 1
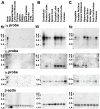
Northern blot analysis of the human γ2, γ3, and γ4 mRNA transcripts. A. Human multiple-tissue northern blots revealed that the γ2, γ3, and γ4 are exclusively detected in the brain. B & C. Brain region blots determined that the γ2 and γ4 are almost ubiquitously expressed, but at differential levels in the same tissues. γ3 is more specifically localized to cerebral cortex, amygdala, caudate nucleus and hippocampus. Size markers were from an RNA ladder provided on the blot by BD Biosciences Clontech and the β-actin control probe results are displayed in the bottom panels.
Figure 2
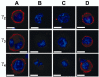
Transient expression of γ2, γ3, and γ4 in COS-7 cells. A. Positive staining for γ2, γ3, or γ4 (red) is strongly localized to the membranes of permeablized cells transfected with the individual γ2, γ3, or γ4 cDNAs. Little immunostaining is observed in the cytoplasm between the membrane and nucleus (blue). B. Non-transfected cells did not stain for γ2, γ3, or γ4 using the Abs generated in this study. C. Cells transfected with γ2, γ3, or γ4 cDNA, but not permeablized during the staining process do not show immunoreactivity for the appropriate anti-γ Abs. D. When co-transfected together with the CaV2.1 and β4 VDCC subunit cDNAs, the expression patterns of γ2, γ3, or γ4 were unaltered. Scale bars in all panels represent 10 μm.
Figure 3
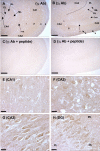
Expression of the γ2, γ3, and γ4 stargazin-like proteins in human cerebellum. A. γ2 immunoreactivity in the molecular layer (m) and part of the adjacent granule cell layer (g) in a cerebellar folium. Strong γ2 immunostaining was seen in the Purkinje cell somata (p) that continued into the dendrites, observed as moderate staining of the molecular layer neuropil. Cell bodies in the molecular layer with positive γ2 immunoreactivity are small interneurons (i). B. In the granule cell layer, the granule cells (g) are moderately stained for γ2 although the strongest immunostaining in this region is actually in the interneurons (i). C. The dentate cerebellar nucleus (dcn), displayed strong γ2 immunostaining in the cell bodies of this nucleus with only weak to moderate staining in the surrounding neuropil. In this particular section, cell nuclei are stained blue by Mayer's hematoxylin counterstain. D. Cerebellar folia displayed little to no γ3 immunostaining in the central white matter (wm) and molecular layer (m). Weak immunostaining was observed in the Purkinje cell bodies and the interneurons of the granule cell layer (g). E. Pre-absorption control, with the peptide immunogen for the γ3 Ab in the adjacent section to Fig. 3D. F. The cell bodies of the Purkinje cells (p) stained strongly for γ4 and this extended well into the Purkinje cell dendrites (d). The surrounding molecular layer neuropil (m) displayed light-moderate staining, as did the granule cell layer (g). Cell bodies in the molecular layer immunoreactive to the γ4 Ab were small interneurons (i). G. Granule cell layer interneurons (i) stained much more strongly for γ4 than the granule cells (g). H. The dcn gave a strong γ4 immunostaining signal in the perisomatic neuropil with only weak staining in the nucleus cell bodies. I. Immunizing peptide pre-absorption control for the γ2 Ab. Pre-absorption of the γ3 or γ4 Abs with their immunizing peptides produced almost identical results. Scale bars in all panels represent 25 μm.
Figure 4
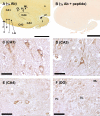
γ2 immunohistochemistry in human hippocampus. A. In the CA1, alveus (a), stratum oriens (o) most adjacent to the pyramidal layer, and the pyramidal layer (p) all stain moderately for γ2. The stratum radiatum (r) and lacunosum-moleculare (l-m) stain much more weakly. B. Strong detection of γ2 protein occurred in the alveus and pyramidal layers of CA2 and CA3 regions. Moderate immunostaining is detected in stratum lucidum (l). Staining of the strata oriens and radiatum was very weak in this region. The dentate gyrus molecular layer (ML) stains moderately for γ2 and the granule layer strongly (GL). γ2 staining is weak or absent in the polymorphic layer (PL). Cells in the CA4 show moderate staining. C and D. Serial sections to those displayed in panels A and B incubated with synthesis peptide pre-absorbed γ2 Ab. E. In the region of the CA1 towards the CA2 the perisomatic staining is light to moderate with similar staining of the cell bodies. F. In the CA2, staining of neuropil and cell bodies is more intense than in CA1. G. Staining of the soma of the CA3 hippocampal neurons is strong with moderate staining of the surrounding neuropil. H. The dentate gyrus shows moderate staining of the molecular layer and dense immunostaining in the granule layer. The polymorphic layer is weakly stained. Scale bar in panels A–D represents 250 μm, in panels E–H 25 μm. The dotted lines in panels A and B represent the border between the CA4 region of the hippocampus and the polymorphic layer of the dentate gyrus.
Figure 5
γ3 immunohistochemistry in human hippocampus. A. The pyramidal cell layer (p) of the CA1 region exhibited moderate to strong staining for γ3. Weak staining was observed in the stratum oriens (o) and stratum radiatum (r). The alveus (a) and lacunosum-moleculare (l–m) are in general, absent of immunoreactivity. B. More intense γ3 immunostaining was observed in the CA2 and CA3 pyramidal cell layers than in CA1. Strong staining was also detected in CA4 cell bodies but surrounding neuropil is devoid of immunoreactivity. C and D. Sections adjacent to those displayed in panels A and B incubated with immunizing peptide-pre-absorbed γ3 Ab. E. Moderate to strong staining of CA1 pyramidal cell bodies and weaker staining in the surrounding neuropil. F. Intense staining of the pyramidal cells of the CA3 with moderate perisomatic staining. G. Cell bodies in the subiculum (Sub) are moderately stained and neuropil staining is very weak. Neuropil staining in panels E, F and G may reflect relative cell densities in each region. H. Granule cells of the dentate gyrus were stained moderately to strongly by the γ3 Ab. The immunoreactivity is mainly in the soma of these cells, but can also be seen in the early branches of the granule cells dendritic trees, which extend into the lightly stained molecular layer. The neuropil of the polymorphic layer is almost devoid of staining. Scale bars in panel A–D represent 250 μm, in panels E–H 25 μm.
Figure 6
γ4 immunohistochemistry in human hippocampus. A. γ4 immunoreactivity was seen throughout the pyramidal cell layers each region of Ammon's horn and the surrounding neuropil with staining most intense in the CA2/3 transition region. Labeled regions are as in figures 4 and 5 with the addition of subiculum (s) choroidal fissure (cf) and fimbria (f). B. Section adjacent to that in panel A incubated with immunizing peptide pre-absorbed γ4 Ab. C. CA1 pyramidal cell bodies are moderately to strongly stained by γ4 Ab with moderate staining in surrounding neuropil. D. Strong staining of pyramidal cells in the CA3 with only slightly weaker immunostaining in the neuropil. E. Staining of CA4 cell bodies is moderate to strong with moderate neuropil staining. F. Although positively stained, the granule neurons of the dentate gyrus do not stain as strongly for γ4 as do pyramidal cells. Scale bars in panel A and B represent 5 mm and in panels C–F 25 μm.
Figure 7
Influence of γ2 and γ4 upon CaV 2.1/β4 ± α2δ2 VDCC currents expressed in Xenopus oocytes. A The peak current-voltage relationships for CaV2.1/β4 (n = 16), CaV2.1/β4/γ2 (n = 14) and CaV2.1/β4/γ4 (n = 11) show no significant differences in any parameters (Table 1). The bottom panels display representative traces recorded from a single oocyte injected with each subunit combination investigated. B. The peak current-voltage relationships for CaV2.1/β4/α2δ2 (n = 43), CaV2.1/β4/α2δ2/γ2 (n = 34) and CaV2.1/β4/α2δ2/γ4 (n = 20) show no significant differences in any parameters (Table 1). In both panels A and B, only representative traces from -50 to +10 mV are displayed for reasons of clarity. C. Mean steady state inactivation data for IBa recorded in 10 mM Ba2+ from Xenopus oocytes injected with CaV2.1/β4/α2δ2 (n = 18) or CaV2.1/β4/α2δγ (n = 18) D. CaV2.1/β4/α2δ2 (n = 20), or CaV2.1/β4/α2δ2/γ4 (n = 20). Co-expression of either γ2 or γ4 produced data almost identical to the control. E. Oocytes injected with CaV2.1/β4 (n = 22), CaV2.1/β4γ2 (n = 16) and CaV2.1/β4γ4 (n = 9) normalized to the maximum current Imax and fitted with a single Boltzmann function. The numerical data for parameters defining the steady-state inactivation relationships are displayed in Table 2.
Similar articles
- Biochemical and anatomical evidence for specialized voltage-dependent calcium channel gamma isoform expression in the epileptic and ataxic mouse, stargazer.
Sharp AH, Black JL 3rd, Dubel SJ, Sundarraj S, Shen JP, Yunker AM, Copeland TD, McEnery MW. Sharp AH, et al. Neuroscience. 2001;105(3):599-617. doi: 10.1016/s0306-4522(01)00220-2. Neuroscience. 2001. PMID: 11516827 - Functional roles of gamma2, gamma3 and gamma4, three new Ca2+ channel subunits, in P/Q-type Ca2+ channel expressed in Xenopus oocytes.
Rousset M, Cens T, Restituito S, Barrere C, Black JL 3rd, McEnery MW, Charnet P. Rousset M, et al. J Physiol. 2001 May 1;532(Pt 3):583-93. doi: 10.1111/j.1469-7793.2001.0583e.x. J Physiol. 2001. PMID: 11313431 Free PMC article. - Loss of calcium channels in the cerebellum of the ataxic and epileptic stargazer mutant mouse.
Leitch B, Shevtsova O, Guévremont D, Williams J. Leitch B, et al. Brain Res. 2009 Jul 7;1279:156-67. doi: 10.1016/j.brainres.2009.04.051. Epub 2009 May 5. Brain Res. 2009. PMID: 19422811 - Differential expression and association of calcium channel subunits in development and disease.
McEnery MW, Vance CL, Begg CM, Lee WL, Choi Y, Dubel SJ. McEnery MW, et al. J Bioenerg Biomembr. 1998 Aug;30(4):409-18. doi: 10.1023/a:1021997924473. J Bioenerg Biomembr. 1998. PMID: 9758336 Review. - Learning from stargazin: the mouse, the phenotype and the unexpected.
Osten P, Stern-Bach Y. Osten P, et al. Curr Opin Neurobiol. 2006 Jun;16(3):275-80. doi: 10.1016/j.conb.2006.04.002. Epub 2006 May 5. Curr Opin Neurobiol. 2006. PMID: 16678401 Review.
Cited by
- Trafficking of neuronal calcium channels.
Weiss N, Zamponi GW. Weiss N, et al. Neuronal Signal. 2017 Feb 20;1(1):NS20160003. doi: 10.1042/NS20160003. eCollection 2017 Feb. Neuronal Signal. 2017. PMID: 32714572 Free PMC article. Review. - Age-related Effects of Heroin on Gene Expression in the Hippocampus and Striatum of Cynomolgus Monkeys.
Choi MR, Jin YB, Bang SH, Im CN, Lee Y, Kim HN, Chang KT, Lee SR, Kim DJ. Choi MR, et al. Clin Psychopharmacol Neurosci. 2020 Feb 29;18(1):93-108. doi: 10.9758/cpn.2020.18.1.93. Clin Psychopharmacol Neurosci. 2020. PMID: 31958910 Free PMC article. - Emerging roles for multifunctional ion channel auxiliary subunits in cancer.
Haworth AS, Brackenbury WJ. Haworth AS, et al. Cell Calcium. 2019 Jun;80:125-140. doi: 10.1016/j.ceca.2019.04.005. Epub 2019 Apr 25. Cell Calcium. 2019. PMID: 31071485 Free PMC article. Review. - CACNG2 polymorphisms associate with chronic pain after mastectomy.
Bortsov AV, Devor M, Kaunisto MA, Kalso E, Brufsky A, Kehlet H, Aasvang E, Bittner R, Diatchenko L, Belfer I. Bortsov AV, et al. Pain. 2019 Mar;160(3):561-568. doi: 10.1097/j.pain.0000000000001432. Pain. 2019. PMID: 30371558 Free PMC article. - Condition-adaptive fused graphical lasso (CFGL): An adaptive procedure for inferring condition-specific gene co-expression network.
Lyu Y, Xue L, Zhang F, Koch H, Saba L, Kechris K, Li Q. Lyu Y, et al. PLoS Comput Biol. 2018 Sep 21;14(9):e1006436. doi: 10.1371/journal.pcbi.1006436. eCollection 2018 Sep. PLoS Comput Biol. 2018. PMID: 30240439 Free PMC article.
References
- Jay SD, Ellis SB, McCue AF, Williams ME, Vedvick TS, Harpold MM, Campbell KP. Primary structure of the γ subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1990;248:490–492. - PubMed
- Powers PA, Liu S, Hogan K, Gregg RG. Molecular characterization of the gene encoding the γ subunit of the human skeletal muscle 1,4-dihydropyridine-sensitive Ca2+ channel (CACNLG), cDNA sequence, gene structure, and chromosomal location. J Biol Chem. 1993;268:9275–9279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases