Role of the gamma-phosphate of ATP in triggering protein folding by GroEL-GroES: function, structure and energetics - PubMed (original) (raw)
Role of the gamma-phosphate of ATP in triggering protein folding by GroEL-GroES: function, structure and energetics
Charu Chaudhry et al. EMBO J. 2003.
Abstract
Productive cis folding by the chaperonin GroEL is triggered by the binding of ATP but not ADP, along with cochaperonin GroES, to the same ring as non-native polypeptide, ejecting polypeptide into an encapsulated hydrophilic chamber. We examined the specific contribution of the gamma-phosphate of ATP to this activation process using complexes of ADP and aluminium or beryllium fluoride. These ATP analogues supported productive cis folding of the substrate protein, rhodanese, even when added to already-formed, folding-inactive cis ADP ternary complexes, essentially introducing the gamma-phosphate of ATP in an independent step. Aluminium fluoride was observed to stabilize the association of GroES with GroEL, with a substantial release of free energy (-46 kcal/mol). To understand the basis of such activation and stabilization, a crystal structure of GroEL-GroES-ADP.AlF3 was determined at 2.8 A. A trigonal AlF3 metal complex was observed in the gamma-phosphate position of the nucleotide pocket of the cis ring. Surprisingly, when this structure was compared with that of the previously determined GroEL-GroES-ADP complex, no other differences were observed. We discuss the likely basis of the ability of gamma-phosphate binding to convert preformed GroEL-GroES-ADP-polypeptide complexes into the folding-active state.
Figures
Fig. 1. AlFx and BeFx complexes support cis folding of the substrate protein rhodanese in the presence of ADP. (A and B) Time course of the recovery of rhodanese activity inside the complex formed between the single-ring GroEL mutant, SR1 and GroES. Binary complexes between urea-denatured rhodanese (1 µM) and SR1 (2 µM) were formed and mixed with GroES (4 µM) and (A) either 3 mM ATP, 5 mM ADP plus 10 mM vanadate, or 5 mM ADP, or (B) 5 mM ADP plus either AlFx [30 mM KF and 3 mM KAl(SO4)2] or BeFx (30 mM KF and 3 mM BeSO4). Enzymatic activity of the rhodanese monomer was assayed directly at the indicated times without disruption of the cis complex. Activity is expressed as a fraction of the final yield of the ATP-driven reaction at 60 min. (C) Ordered addition of the AlFx γ-phosphate analogue to a preformed, folding-inactive SR1–GroES–ADP–rhodanese complex also triggers rhodanese folding. A binary complex was formed between urea-denatured rhodanese (1 µM) and SR1 (2 µM), and mixed with GroES (4 µM) and 5 mM ADP. After 30 min, AlFx [30 mM KF and 3 mM KAl(SO4)2] was added to the mixture. Aliquots were taken for enzyme assay at the indicated times both before and after AlFx addition. In each panel, the results from a representative experiment are presented.
Fig. 2. Rhodanese is released into the cis cavity following addition of GroES and either ATP or ADP·AlFx to SR1–rhodanese. Binary complexes between urea-denatured, pyrene-labelled rhodanese and SR1 were mixed (1:1) in a stopped-flow apparatus with solutions containing 10 µM GroES and either 3 mM ATP (blue trace), 5 mM ADP (black trace) or 5 mM ADP and AlFx [3 mM KAl(SO4)2 and 30 mM KF] (green trace). The AlFx mixture alone was also mixed (1:1) in the stopped-flow with a solution of preformed SR1–rhodanese-GroES–ADP complex (red trace). The anisotropy of the pyrene label, reflecting the mobility of the polypeptide and its release from the cavity walls, was monitored as a function of time after mixing. Traces represent summations of 10−15 runs.
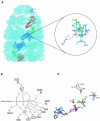
Fig. 3. Crystallographic model of the GroEL–GroES–ADP·AlF3 complex and illustration of the nucleotide-binding pocket. (A) Unbiased electron density map (light blue) calculated with coefficients _F_obsexp(iαave) where the phases αave result from 7-fold NCS averaging, density modification, and phase extension starting from random phases. The map is contoured at 1σ and was calculated using all diffraction data between 50.0 Å and 2.8 Å resolution. A Cα trace is shown for the entire complex, with subunits from a single protomer highlighted as follows: the GroES subunit is shown in magenta; and the apical, intermediate and equatorial domains are shown in red, green and blue, respectively. (Inset) σA-weighted |_F_o|−|_F_c| electron density map contoured at 3.5 σ showing difference density for both AlF3, bound in the γ-phosphate position of the ATP binding pocket in the cis ring of GroEL−GroES, and a coordinating K+ metal ion. The protein is shown as a skeletal model, with relevant residues in the equatorial and intermediate domains coloured blue and green, respectively, ADP is white, the Mg2+ is a red sphere, K+ is a yellow sphere, and the trigonal AlF3 is shown as an orange (aluminium) and green (fluorine) ball-and-stick model. Interactions are indicated by grey dashed lines. (B) Schematic representation of the coordination of aluminium fluoride in the cis active site of GroEL–GroES. Interactions are shown as dotted lines. The catalytic water could not be unambiguously assigned, probably owing to the limited resolution of the diffraction data, and was not included in the final model, but is shown here to illustrate its likely interaction partners based on results from (C), a least-squares superposition of conserved interactions in the nucleotide binding site in GroEL–GroES and the thermosome complexed with ADP·AlF3 (Ditzel et al., 1998). The thermosome-derived side-chains ADP, AlF3, water (Wat) and Mg++ are shown in light blue.
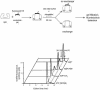
Fig. 4. Aluminium fluoride binding stabilizes the cis ADP complex, mimicking the effects of binding of intact ATP. The stability of the cis complex was probed by perturbation with a chaotrope (schematic). SR1 was incubated for 30 min at 25°C with fluorescently labelled GroES in the presence of either 5 mM ADP, 5 mM AMP-PNP or 3 mM ADP plus 3 mM KAl(SO4)2 and 30 mM KF; SR398 was similarly incubated with 3 mM ATP. Complexes were exposed to 0.35 M GuHCl for 30 min in the presence of excess unlabelled GroES competitor, then subjected to gel filtration with fluorescence detection. The results of a representative experiment are presented.
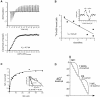
Fig. 5. Energetics of forming a folding-active cis complex in SR1, with discrete contributions of consecutive ADP, GroES and aluminium fluoride binding steps. (A) Isothermal calorimetric titration of SR1 with ADP at 25°C. (Upper panel) heat change produced at each injection of ADP; (bottom panel) the integrated data from the upper trace: the line was generated by the fit to the binding equation. A representative experiment is shown. ADP binding to SR1 is exothermic with a _K_D of 32.7 ± 1.4 µM (n = 3) and a stoichiometry of 7 ADP molecules/SR1 heptamer. (B) Affinity of SR1–ADP7 for GroES measured by a Hummel-Dreyer experiment. SR1 was chromatographed on a gel filtration column equilibrated with various amounts of [35S]GroES and 5 mM ADP; the inset shows a typical elution profile, here for 1 µM [35S]GroES. The area at the SR1 elution position was used to calculate the amount of bound GroES. Bound versus bound/free GroES was plotted (main figure), and the dissociation constant was determined from the slope of the line (−_K_D) to be 0.40 µM in two experiments. (C) Measurement of AlFx binding to SR1–ES–ADP by competition with [7Be]Fx. Main panel: the binding of [7Be]Fx to SR1–ES–ADP was measured by a spin-column assay (Materials and methods), and observed to be saturable and apparently non-cooperative (_K_D = 30 µM). (Inset) the effect of AlFx on [7Be]Fx binding is shown in an Eadie–Hofstee plot of the data from parallel binding experiments, one without and one with 50 µM AlFx. The maximal amount of [7Be]Fx bound (_y_-intercept) was unaltered in the presence of AlFx, demonstrating competitive binding between [7Be]Fx and AlFx. This allows the calculation of the _K_D for aluminium fluoride binding of 16 ± 1 µM (n = 3) (see Supplementary Materials and methods). (D) Estimated free energy transitions during activation of SR1. The standard free energy drops proceed in three stages [ADP binding (−42.7 kcal/mol), GroES binding (−8.7 kcal/mol) and aluminium fluoride binding (−45.8 kcal/mol)] and are computed at 25°C from the corresponding values of _K_D.
Similar articles
- Substrate polypeptide presents a load on the apical domains of the chaperonin GroEL.
Motojima F, Chaudhry C, Fenton WA, Farr GW, Horwich AL. Motojima F, et al. Proc Natl Acad Sci U S A. 2004 Oct 19;101(42):15005-12. doi: 10.1073/pnas.0406132101. Epub 2004 Oct 12. Proc Natl Acad Sci U S A. 2004. PMID: 15479763 Free PMC article. - Characterization of the active intermediate of a GroEL-GroES-mediated protein folding reaction.
Weissman JS, Rye HS, Fenton WA, Beechem JM, Horwich AL. Weissman JS, et al. Cell. 1996 Feb 9;84(3):481-90. doi: 10.1016/s0092-8674(00)81293-3. Cell. 1996. PMID: 8608602 - Mechanism of chaperonin action: GroES binding and release can drive GroEL-mediated protein folding in the absence of ATP hydrolysis.
Hayer-Hartl MK, Weber F, Hartl FU. Hayer-Hartl MK, et al. EMBO J. 1996 Nov 15;15(22):6111-21. EMBO J. 1996. PMID: 8947033 Free PMC article. - Structure and function in GroEL-mediated protein folding.
Sigler PB, Xu Z, Rye HS, Burston SG, Fenton WA, Horwich AL. Sigler PB, et al. Annu Rev Biochem. 1998;67:581-608. doi: 10.1146/annurev.biochem.67.1.581. Annu Rev Biochem. 1998. PMID: 9759498 Review. - GroEL and the GroEL-GroES Complex.
Ishii N. Ishii N. Subcell Biochem. 2017;83:483-504. doi: 10.1007/978-3-319-46503-6_17. Subcell Biochem. 2017. PMID: 28271487 Review.
Cited by
- Low-temperature features of the psychrophilic chaperonin from Pseudoalteromonas haloplanktis.
Hertle E, Ursinus A, Martin J. Hertle E, et al. Arch Microbiol. 2024 Jun 11;206(7):299. doi: 10.1007/s00203-024-04019-y. Arch Microbiol. 2024. PMID: 38861015 Free PMC article. - Generic nature of the condensed states of proteins.
Fuxreiter M, Vendruscolo M. Fuxreiter M, et al. Nat Cell Biol. 2021 Jun;23(6):587-594. doi: 10.1038/s41556-021-00697-8. Epub 2021 Jun 9. Nat Cell Biol. 2021. PMID: 34108660 Review. - Mechanism of nucleotide sensing in group II chaperonins.
Pereira JH, Ralston CY, Douglas NR, Kumar R, Lopez T, McAndrew RP, Knee KM, King JA, Frydman J, Adams PD. Pereira JH, et al. EMBO J. 2012 Feb 1;31(3):731-40. doi: 10.1038/emboj.2011.468. Epub 2011 Dec 23. EMBO J. 2012. PMID: 22193720 Free PMC article. - GroEL-mediated protein folding: making the impossible, possible.
Lin Z, Rye HS. Lin Z, et al. Crit Rev Biochem Mol Biol. 2006 Jul-Aug;41(4):211-39. doi: 10.1080/10409230600760382. Crit Rev Biochem Mol Biol. 2006. PMID: 16849107 Free PMC article. Review. - The newly discovered Q motif of DEAD-box RNA helicases regulates RNA-binding and helicase activity.
Cordin O, Tanner NK, Doère M, Linder P, Banroques J. Cordin O, et al. EMBO J. 2004 Jul 7;23(13):2478-87. doi: 10.1038/sj.emboj.7600272. Epub 2004 Jun 17. EMBO J. 2004. PMID: 15201868 Free PMC article.
References
- Alberts B. (1998) The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell, 92, 291–294. - PubMed
- Brunger A.T. et al. (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. - PubMed
- Burston S.G., Ranson,N.A. and Clarke,A.R. (1995) The origins and consequences of asymmetry in the chaperonin reaction cycle. J. Mol. Biol., 249, 138–152. - PubMed
- Cliff M.J., Kad,N.M., Hay,N., Lund,P.A., Webb,M.R., Burston,S.G. and Clarke,A.R. (1999) A kinetic analysis of the nucleotide-induced allosteric transitions of GroEL. J. Mol. Biol., 293, 667–684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials