Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44 - PubMed (original) (raw)
Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44
Tiziana Anelli et al. EMBO J. 2003.
Abstract
Formation of disulfide bonds, an essential step for the maturation and exit of secretory proteins from the endoplasmic reticulum (ER), is controlled by specific ER-resident enzymes. A pivotal element in this process is Ero1alpha, an oxidoreductin that lacks known ER retention motifs. Here we show that ERp44 mediates Ero1alpha ER localization through the formation of reversible mixed disulfides. ERp44 also prevents the secretion of an unassembled cargo protein with unpaired cysteines. We conclude that ERp44 is a key element in thiol-mediated retention. It might also favour the maturation of disulfide-linked oligomeric proteins and their quality control.
Figures
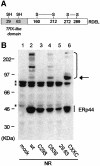
Fig. 1. ERp44 forms mixed disulfides with Ero1α and numerous other proteins. (A) Lysates from HepG2, SK-N-BE and HeLa cells were resolved under reducing (R) or non-reducing (NR) conditions (8.8% SDS–PAGE), blotted and decorated with a monoclonal antibody (36C9) against ERp44. The migration of reduced and oxidized ERp44 monomers and covalent complexes is indicated on the left hand margin. (B) HeLa cells transiently transfected with HA-ERp44 were treated with DTT for 15 min at 37°C at the indicated concentrations prior to lysis. Aliquots were resolved under non-reducing conditions (10% SDS–PAGE), blotted and decorated with monoclonal anti-HA antibodies. Asterisks denote background bands, as indicated by the reactivity of the antibody on a lysate of mock transfected cells (lane 0). (C) HeLa cells co-transfected with HA-ERp44 and Ero1α-myc were processed as in (B). The identity of ERp44/Ero1α complexes was confirmed by decorating the blot shown in (C) with anti-myc antibodies (not shown).
Fig. 2. ERp44 forms mixed disulfides with endogenous proteins via C29 in the trx-like domain. (A) Schematic diagram of ERp44. The redox status and pairing of the cysteines was determined by mass spectrometry analyses. (B) Lysates from HeLa cells transfected with the indicated HA-ERp44 mutants were resolved under non-reducing (NR) conditions, blotted and decorated with anti-HA. The arrow on the right points to a complex the abundance of which increases in both C63S and CxxC mutants: mass spectrometry analyses confirmed that this consists mainly if not only of ERp44 homodimers. Complexes between ERp44 and endogenous HeLa cargo proteins are indicated on the right hand margin. Asterisks denote background bands (see Figure 1B).
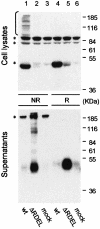
Fig. 3. The C-terminal RDEL motif mediates intracellular retention of ERp44. Aliquots from the lysates (top panel) and the anti-HA immunoprecipitated material from the supernatants (bottom panel) of HeLa cells expressing ERp44 (wt) or a mutant lacking the C-terminal RDEL motif (ΔRDEL) were resolved under reducing (R) or non-reducing (NR) conditions, and processed as in Figure 2B. Asterisks denote background bands.
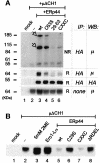
Fig. 4. ERp44 mediates the intracellular retention of Ero1α. HeLa cells were co-transfected with Ero1α-myc alone or with different HA-ERp44 mutants as indicated. Cells transfected only with the empty vector were used as control (mock). The supernatants of 3.5 × 106 cells cultured over night in Optimem (SN, upper panel) were precipitated with Concanavalin A–Sepharose (ConA) or anti-HA, as indicated. Aliquots of the lysates corresponding to 1.5 × 106 cells were subjected to immunoprecipitation with anti-HA and resolved under reducing conditions (lower panel). Blots were sequentially decorated with anti-myc (Ero1α) and anti-HA (ERp44) antibodies.
Fig. 5. ERp44 binds covalently to unassembled µ chains and retains them intracellularly. (A) ERp44 forms mixed disulfides with µΔCH1. Lysates of 3 × 106 HeLa cells transfected with µΔCH1 alone or with HA-ERp44 (wt or mutated), or with the empty vector (mock) were subjected to immunoprecipitation with anti-HA, resolved under non-reducing or reducing conditions and immunoblotted with anti-µ and anti-HA antibodies, as indicated. Based on their mobility, the two main bands recognized by the anti-µ antibody likely correspond to µΔCH1 monomers and dimers covalently linked to HA-ERp44 (see arrowheads). As a control of the amount of µΔCH1 in each sample, lysates of 1.2 × 105 cells of each transfection were resolved under reducing conditions and decorated with anti-µ antibody. Note that the mutant CxxC binds less µΔCH1 than wt molecules, at steady state. (B) The supernatants of HeLa cells transfected as indicated were collected after 4 h of culture in Optimem, concentrated with ConA Sepharose and resolved under reducing conditions; blots were decorated with anti-μ. Lane 3 shows the material precipitated from the supernatant of cells treated with 5 mM 2ME for 4 h.
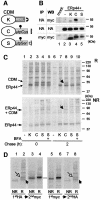
Fig. 6. ERp44 binds to C575 in the µtp. (A) Scheme of the cathepsin D chimeras utilized (Fra et al., 1993; Pelham and Munro, 1993; Isidoro et al., 1996). C-terminally myc-tagged cathepsin D constructs were further extended with SEKDEL (K) or the 20 residue µtp with a cysteine (C) or a serine (S) in the penultimate position (Fra et al., 1993). The µtp contains an _N_-glycan. As described previously, CDMµtpSer is transported to the Golgi and in part secreted, while CDMK and CDMtpCys are localized in the ER, the former because of its KDEL-dependent retrieval and the latter because of thiol-mediated retention. Only CDMK accumulates intracellularly with phosphorylated glycans (Isidoro et al., 1996). (B) Interaction between ERp44 and CDM chimeras at steady state. Lysates of 1 × 106 HeLa cells transfected with the vector alone (mock), or with HA-ERp44 alone or with different CDM chimeras were subjected to immunoprecipitation with anti-HA or with anti-myc, as a control. The immunoprecipitated material was then resolved in SDS–PAGE under reducing conditions, and decorated with anti-myc or anti-HA antibodies, as indicated. Only a very little amount of CDMK and CDMµtpSer co-precipitated with ERp44 when compared with CDMµtpCys. Note that the µtp confers higher electrophoretic mobility to the chimeras, also owing to the presence of a glycosylation site [also see (A)]. (C) HeLa cells co- transfected with HA-ERp44 and cathepsin D chimeras as indicated were pulsed for 15 min with [35S]amino acids and chased for 0 (lanes 1–5) or 2 h (lanes 6–10) with or without BFA (1 µg/ml). The anti-HA immunoprecipitated materials obtained from cell lysates were resolved under reducing (R, top panel) or non-reducing (NR, bottom panel) conditions. While at the beginning of the chase all CDM chimeras co-precipitate with ERp44, after 2 h chase only CDMµtpCys remains covalently associated with it (lane 8, see arrows). The additional bands present in lanes 2–5 probably correspond to other HeLa endogenous proteins that associate with HA-ERp44, and are released with different kinetics (compare lanes 7–10). (D) Only a fraction of CDMµtpCys is bound to ERp44 at steady state. Lysates of HeLa cells co-transfected with HA-ERp44 and CDMµtpCys, and chased for 2 h after a 15 min pulse of radioactive aminoacids [see (B)] were subjected to immunoprecipitation (IP) with anti-HA or with anti-myc; the leftover of these first IPs were subsequently subjected to IP with anti-myc or anti-HA, respectively. Note that anti-myc antibodies do not co-precipitate ERp44, probably because the myc epitope is masked in the ERp44–CDM complexes. About 10% of CDMµtpCys is bound to ERp44 after 2 h of chase. Closed and open arrows indicate reduced ERp44 and CDM, respectively.
Similar articles
- ERp44 mediates a thiol-independent retention of formylglycine-generating enzyme in the endoplasmic reticulum.
Mariappan M, Radhakrishnan K, Dierks T, Schmidt B, von Figura K. Mariappan M, et al. J Biol Chem. 2008 Mar 7;283(10):6375-83. doi: 10.1074/jbc.M709171200. Epub 2008 Jan 4. J Biol Chem. 2008. PMID: 18178549 - The pivotal role of ERp44 in patrolling protein secretion.
Tempio T, Anelli T. Tempio T, et al. J Cell Sci. 2020 Nov 10;133(21):jcs240366. doi: 10.1242/jcs.240366. J Cell Sci. 2020. PMID: 33173013 Review. - Two conserved cysteine triads in human Ero1alpha cooperate for efficient disulfide bond formation in the endoplasmic reticulum.
Bertoli G, Simmen T, Anelli T, Molteni SN, Fesce R, Sitia R. Bertoli G, et al. J Biol Chem. 2004 Jul 16;279(29):30047-52. doi: 10.1074/jbc.M403192200. Epub 2004 May 10. J Biol Chem. 2004. PMID: 15136577 - Dynamic retention of Ero1alpha and Ero1beta in the endoplasmic reticulum by interactions with PDI and ERp44.
Otsu M, Bertoli G, Fagioli C, Guerini-Rocco E, Nerini-Molteni S, Ruffato E, Sitia R. Otsu M, et al. Antioxid Redox Signal. 2006 Mar-Apr;8(3-4):274-82. doi: 10.1089/ars.2006.8.274. Antioxid Redox Signal. 2006. PMID: 16677073 - The physiological functions of mammalian endoplasmic oxidoreductin 1: on disulfides and more.
Ramming T, Appenzeller-Herzog C. Ramming T, et al. Antioxid Redox Signal. 2012 May 15;16(10):1109-18. doi: 10.1089/ars.2011.4475. Epub 2012 Feb 15. Antioxid Redox Signal. 2012. PMID: 22220984 Review.
Cited by
- ERp44/CG9911 promotes fat storage in Drosophila adipocytes by regulating ER Ca2+ homeostasis.
Bi Y, Chang Y, Liu Q, Mao Y, Zhai K, Zhou Y, Jiao R, Ji G. Bi Y, et al. Aging (Albany NY). 2021 May 24;13(11):15013-15031. doi: 10.18632/aging.203063. Epub 2021 May 24. Aging (Albany NY). 2021. PMID: 34031268 Free PMC article. - Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha.
Qiang L, Wang H, Farmer SR. Qiang L, et al. Mol Cell Biol. 2007 Jul;27(13):4698-707. doi: 10.1128/MCB.02279-06. Epub 2007 Apr 23. Mol Cell Biol. 2007. PMID: 17452443 Free PMC article. - Endoplasmic reticulum-mediated protein quality control in Arabidopsis.
Liu Y, Li J. Liu Y, et al. Front Plant Sci. 2014 Apr 30;5:162. doi: 10.3389/fpls.2014.00162. eCollection 2014. Front Plant Sci. 2014. PMID: 24817869 Free PMC article. Review. - Introducing Thioredoxin-Related Transmembrane Proteins: Emerging Roles of Human TMX and Clinical Implications.
Matsuo Y. Matsuo Y. Antioxid Redox Signal. 2022 May;36(13-15):984-1000. doi: 10.1089/ars.2021.0187. Epub 2021 Dec 7. Antioxid Redox Signal. 2022. PMID: 34465218 Free PMC article. Review. - The redox proteome.
Go YM, Jones DP. Go YM, et al. J Biol Chem. 2013 Sep 13;288(37):26512-20. doi: 10.1074/jbc.R113.464131. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861437 Free PMC article. Review.
References
- Alberini C.M., Bet,P., Milstein,C. and Sitia,R. (1990) Secretion of immunoglobulin M assembly intermediates in the presence of reducing agents. Nature, 347, 485–487. - PubMed
- Benedetti C., Fabbri,M., Sitia,R. and Cabibbo,A. (2000) Aspects of gene regulation during the UPR in human cells. Biochem. Biophys. Res. Commun., 278, 530–536. - PubMed
- Cabibbo A., Pagani,M., Fabbri,M., Rocchi,M., Farmery,M.R., Bulleid,N.J. and Sitia,R. (2000) ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem., 275, 4827–4833. - PubMed
- Ellgaard L. and Helenius,A. (2001) ER quality control: towards an understanding at the molecular level. Curr. Opin. Cell Biol., 13, 431–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous