MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1 - PubMed (original) (raw)
MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1
Thomas Timm et al. EMBO J. 2003.
Abstract
MARK, a kinase family related to PAR-1 involved in establishing cell polarity, phosphorylates microtubule-associated proteins (tau/MAP2/MAP4) at KXGS motifs, causes detachment from microtubules, and their disassembly. The sites are prominent in tau from Alzheimer's disease brains. We studied the activation of MARK and identified the upstream kinase, MARKK, a member of the Ste20 kinase family. It phosphorylates MARK within the activation loop (T208 in MARK2). A fraction of MARK in brain tissue is doubly phosphorylated (at T208/S212), reminiscent of the activation of MAP kinase; however, the phosphorylation of the second site in MARK (S212) is inhibitory. In cells the activity of MARKK enhances microtubule dynamics through the activation of MARK and leads to phosphorylation and detachment of tau or equivalent MAPs from microtubules. Overexpression of MARK eventually leads to microtubule breakdown and cell death, but in neuronal cells the primary effect is to allow the development of neurites during differentiation.
Figures
Fig. 1. Diagram of MARK1-4 (DDBJ/EMBL/GenBank accession Nos Z83868, Z83869, AF240782 and AY057448). Domains: N = header, C = catalytic, T = membrane-targeting, UBA = ubiquitin-associated, S = spacer, T = tail.
Fig. 2. Activation of MARK2. (A) Protein and activity profile of G200 gel filtration column. Fractions of brain extract were tested for the activation of MARK to phosphorylate the TR1 peptide. Most of the protein elutes around fraction 10 (bottom), but the activity peaks in fractions 13–14 (top, arrow). Calibration of column is shown as dotted line (catalase, ferritin, aldolase, serum albumin, ovalbumin). (B) Activation of MARK2 by MARK-activating factor. Bottom: recombinant MARK2 alone phosphorylates the TR1 peptide at a constant low rate (55 nmol/min/mg). With MAF (top) the activity of MARK2 increases ∼10-fold. (C) Phosphorylation of MARK2 increases with activation by MAF. Top left: SDS gel showing the _M_r shift (from 88 to 97 kDa) during activation by MAF. Top right: MARK2 alone shows no _M_r shift, despite some autophosphorylation. Bottom left: Autoradiogram of gel showing the increase in phosphate bound to MARK2, mostly at T208 (see below). Bottom right: MARK2 alone shows little autophosphorylation (outside the catalytic domain, data not shown). (D) Lanes 1 and 2, autoradiograms of MARK2 in active and inactive states. Activity was blocked by covalent modification of ATP binding site by FSBA. Lanes 3 and 4, incorporation of phosphate into active and inactive MARK2 by MAF, illustrating that MAF contains a kinase independently of the activity of MARK2. Lane 5, control, MAF alone shows no phosphate incorporation at the position of MARK2.
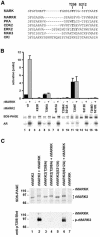
Fig. 3. Sequence and domain structure of MARKK. (A) Identification of MARKK. Lanes 1–4 show SDS gel of fractions 11, 14, 16 (with FSBA), and 14 (control without FSBA) from G200 column (fraction 14 contains the highest activity, see Figure 2A). Lanes 5–8 are blots with an antibody against FSBA. Only one band at 134 kDa is labeled in the active fraction (lane 6, fraction 14); the control without FSBA shows no signal (lane 8). For comparison, lane 9 shows an autoradiogram of an active fraction (same sample as in lane 4) with a band of active MARKK at Mapp= 134 kDa, labeled because of autophosphorylation. (B) Sequence of MARKK from a rat brain cDNA library. Peptides identified by sequencing and mass spectrometry are underlined. The sequence is equivalent to that of TAO-1 (DDBJ/EMBL/GenBank accession no. AF084205) or the human sequence KIAA1361 (AB037782). The three bold peptides are unique for TAO-1 and distinguish the kinase from PSK/TAO-2 and JIK. (C) Domain structure of MARKK/TAO-1 and comparison with other kinases of high homology (JIK and PSK) in the Ste20 family. N = N-terminal header domain, C = catalytic domain, SBD = substrate binding domain, S = spacer domain, T = tail domain. Extended coiled-coil sequences are predicted in the spacer and tail domains (S430-A630, K730-F900). The substrate binding domain of PSK/TAO-2 binds to the N-terminal header domain of its substrate MKK3, the kinase upstream of p38 MAP kinase. However, it is unclear whether an analogous interaction occurs with MARKK and MARK since the corresponding domains are poorly conserved.
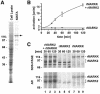
Fig. 4. Activity of recombinant MARKK. (A) Expression of recombinant MARKK (His10-tagged at the N-terminus) in Sf9 cells. Lane 1, SDS gel of Sf9 cell lysate, lane 2, protein retained on Ni-NTA column, showing enrichment of MARKK at Mapp = 134 kDa (slightly larger than the predicted 119 kDa). (B) Activation of MARK2 by recombinant MARKK, measured by the phosphorylation of the TR1 peptide by MARK2. Bottom curve (squares), recombinant MARK2 alone phosphorylates the peptide at a constant low rate. MARKK activates MARK2 >16-fold after 2 h (top curve, circles). (C) Phosphorylation of MARK2 increases with activation by MARKK. Top left (lanes 1–3), SDS gel showing the gradual shift of MARK2 in _M_r (from 88 to 97 kDa) during activation by MAF. Top middle (lanes 4–6), MARK2 alone shows no shift in _M_r, despite some autophosphorylation. Top right (lanes 7–9), MARKK alone shows no shift in _M_r either. Bottom left, autoradiogram of gel showing the increase in phosphate bound to MARK2, mostly at T208 (see below). Bottom middle, MARK2 alone shows little autophosphorylation (at sites outside the catalytic domain; data not shown). Bottom right, MARKK shows some autophosphorylation (note that the lower band comigrating with MARK2 is a coincidence and probably due to degradation of MARKK).
Fig. 5. Regulation of MARK. (A) Activation loop of MARK2 and other kinases. Known phosphorylation sites are in bold. (B) Influence of activation loop on MARK2 activity. Top panel, bar 1, MARK2 alone has a low basal activity of 55 nmol/min/mg. Bar 2, activation by MARKK is 10-fold. Bars 3 and 4, the K82R mutant has no activity and cannot be activated. Bars 5 and 6, the T208A mutant has the same activity as wild type, irrespective of MARKK, indicating that the phosphorylation of T208 is important for activation. Bars 7–10, the S212A mutant and the T208A/S212A mutant of MARK2 show no activity at all, indicating that S212 is important for basal activity, independently of whether T208 is phosphorylated by MARKK or not. Bars 11 and 12, the T208E mutant has a 4-fold higher activity than the wild type but cannot be activated further by MARKK. Bars 13–16, the S212E mutant and the T208E/S212E mutant of MARK2 show no activity. Middle panel, SDS gel shows the shift in _M_r of all MARK2 mutants after phosphorylation with MARKK as well as the wild-type MARK2. The T208E mutant has a lower electrophoretic mobility even without phosphorylation (lane 11). Bottom panel, autoradiograms showing that autophosphorylation corresponds to activity (odd lanes). All proteins become phosphorylated upon incubation with MARKK (even lanes). This indicates at least one phosphorylation site outside the regulatory loop but with no influence on activation. (C) Phosphorylation of MARK2 and mutants (T208A or S212A) during activation by MARKK. Top, SDS gel; bottom, blot with antibody against phospho-Thr. Odd lanes without MARKK; even lanes with activation by MARKK. Lane 1, MARK2 alone. Lane 2, MARKK phosphorylates MARK2 with a shift in _M_r (top), and with a prominent phospho-Thr site recognized by the pT208 antibody. Lane 3, T208A mutant of MARK2. Lane 4, MARKK causes an _M_r shift of MARK2-T208A (top), but there is no phospho-Thr reaction, concomitant with the loss of activation [see (B) above]. It also shows that T208 is not responsible for the _M_r shift. Lane 5, S212A mutant of MARK2. Lane 6, phosphorylation by MARKK of MARK2-S212A creates the phospho-Thr epitope at T208 (but without activity). There is an _M_r shift that occurs independently of S212. Lane 7, Control, MARKK alone shows no signal with the antibody against phospho-T208.
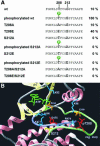
Fig. 6. Regulation of MARK. (A) Sequence F202-E219 around the activation loop of MARK2, with T208 and S212 highlighted. The wild-type sequence allows only basal activity (10%) which rises 10-fold upon phosphorylation of T208 by MARKK. Mutant T208A allows only basal activity, T208E shows partial activation. Phosphorylation of S212 is not required for activation, but any mutation of S212 (A or E) destroys activity, showing that S212 must be present for activity while mutants of S212 are inactive even when T208 is phosphorylated. (B) Structural model of the regulatory loop of MARK2 using PKA as a template (SWISSMODEL,
http://www.expasy.ch/swissmod/SWISS-MODEL.html
). Catalytic (violet) and activation loops (light green) lie between the small lobe (mainly β-sheets, light yellow) and the large lobe (mainly α-helices, pink). Phospho-T208 (red) contacts K96, R174 (light blue) and E199 (orange) via hydrogen bonds stabilizing the regulatory loop. S212 (yellow) forms a hydrogen bond with K177 (light blue) in the catalytic loop which also stabilizes the γ-phosphate of ATP (green) during the phospho-transfer and is conserved in S/T-kinases. The catalytic D175 (red) is stabilized by N180 (light blue). K82 (light blue) fixes the α- and β-phosphates of ATP, together with the Mg2+-binding D193 (orange). Note that in PKA and MAP kinase the phosphorylation corresponding to T208 stabilizes the activation loop and opens the substrate pocket (by interacting with the residues corresponding to K96, R174 and E199 in MARK, Johnson et al., 1996), whereas the conserved DFG motif (193–195) and S212 form fixed points for anchoring this flexible loop (contributing a hydrogen bond with K177). In this scheme, the phosphorylation of S212 would be inhibitory because it would disrupt the fixation of the loop. It is also possible that the Glu in this postion interferes with the binding of the substrate to the catalytic cleft (Scott et al., 2002). Mutation of the equivalent to S212 in JIK results in a dramatic decrease of activity (Tassi et al., 1999). The doubly phosphorylated peptide in brain-derived MARK2 probably come from an inactive fraction. The kinase responsible for phospho-S212 is unknown, but we note that none of the kinases applied in combination with MARKK was able to phosphorylate S212 in MARK2, and there was no change in activity.
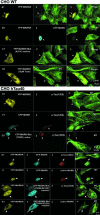
Fig. 7. Active MARKK in cells destroys microtubules via activation of MARK and phosphorylation of Tau. (A) CHO cell transfected with YFP-MARKK, fixed and observed by confocal microscopy (A1, arrow), staining of microtubules with antibody YL1/2 and Cy5-secondary antibody (A2), merged images (A3). Note that the transfected cell (arrow) lost its microtubule network, rounded up and appears smaller. (B) Cell co-transfected with YFP-MARKK (B1, arrow) and CFP-MARK (B2) or staining of microtubules with YL1/2 (B3). Note the shape change and destruction of microtubules in the co-transfected cell, similar to (A). (C) CHO cell transfected with inactive YFP-MARKKK57A imaged as above (C1, YFP fluorescence, C2 microtubules, C3 merged). The transfected cell (left, arrow) retains its microtubule network. (D) Cell transfected with YFP-MARKK (D1, arrow), and microtubules stabilized by 10 µM taxol (D2), merged images (D3). Note that taxol prevents the destabilization of microtubules by MARKK. (E) Cell stably transfected with tau and transiently transfected with YFP-MARKK. Staining of YFP-MARKK (E1, arrow), phospho-tau (KXGS motifs) by antibody 12E8 (TRITC) (E2), microtubules (E3). Note that elevated tau stabilizes microtubules against the effect of MARKK. (F) Cell stably transfected with tau and transiently transfected with CFP-MARK. Staining of CFP-MARK (F1, arrow), phospho-tau (KXGS motifs) (F2), microtubules (F3). Note that elevated tau stabilizes microtubules against the effect of MARK. (G) Cell stably transfected with tau and transiently transfected with CFP-MARKT208E. Constitutively active MARK (G1, arrow) phophorylates tau at KXGS motifs (G2), and destroys microtubules (G3). (H) Cell stably transfected with tau and transiently co-transfected with YFP-MARKK and CFP-MARK. Staining of YFP–MARKK (H1, arrow), CFP-MARK (H2), phospho-tau (KXGS motifs) (H3), and microtubules (H4). Note that the combined effect of MARKK and MARK destabilizes microtubules in spite of tau. (I) Experiment similar to (H) but highlighting active MARK by the antibody SA6941-TRITC against the phosphorylated activation loop (I3, arrow). YFP–MARKK (I1), CFP–MARK (I2), active MARK (TRITC) (I3), and microtubules (Cy5) (I4). (J) Cell stably transfected with tau and transiently co-transfected with YFP-MARKK and CFP-MARK-mutant (T208A, S212A). Staining as in (H). Microtubules remain intact because the MARK mutant cannot be activated. (K) Experiment similar to (J), i.e. cotransfection with YFP-MARKK (K1, arrow), CFP-MARK mutant (K2), and staining with antibody against active MARK (K3).
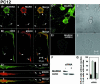
Fig. 8. (A) PC12 cells differentiated with NGF (72 h, 100 ng/ml) and immunostained for MARKK (A1, antibody TAO-1), MARK (A2, antibody SA2118), and merged image (A3). Note that endogenous MARKK and MARK colocalize, notably beneath the plasma membrane and at growth cones (arrows). (B) PC12 cells differentiated as above, immunostained for phospho-KXGS-tau (B1, antibody 12E8), MARK (B2), and merge (B3). Note that endogenous phospho-KXGS-tau colocalizes with MARK, especially near the plasma membrane and the growth cones. (C) PC12 cells differentiated as above and immunostained for actin (C1, oregon-green-phalloidin), MARK (C2), and merge (C3). Note that endogenous MARK colocalizes with actin at growth cones. (D) PC12 cells differentiated with NGF (48 h, 100 ng/ml). D1, mock transfected cell, stained for endogenous MARKK. D2, phase image. (E) PC12 cells transfected with RNAi against MARKK exposed to NGF as above. Note that MARKK is largely suppressed (E1), and the cells cannot differentiate (phase image, E2). (F) Western blot of PC12 cells after NGF-differentiation, mock-transfected (left) or with RNAi against MARKK (right). Note that expression of MARKK is suppressed. (G) Quantification of effects of RNAi against MARKK (see D and E). The fraction of cells with neurites decreases from ∼80% to 20% upon treatment with RNAi.
Similar articles
- Signaling from MARK to tau: regulation, cytoskeletal crosstalk, and pathological phosphorylation.
Timm T, Matenia D, Li XY, Griesshaber B, Mandelkow EM. Timm T, et al. Neurodegener Dis. 2006;3(4-5):207-17. doi: 10.1159/000095258. Neurodegener Dis. 2006. PMID: 17047359 - MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons.
Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E. Mandelkow EM, et al. J Cell Biol. 2004 Oct 11;167(1):99-110. doi: 10.1083/jcb.200401085. Epub 2004 Oct 4. J Cell Biol. 2004. PMID: 15466480 Free PMC article. - The tau of MARK: a polarized view of the cytoskeleton.
Matenia D, Mandelkow EM. Matenia D, et al. Trends Biochem Sci. 2009 Jul;34(7):332-42. doi: 10.1016/j.tibs.2009.03.008. Epub 2009 Jun 24. Trends Biochem Sci. 2009. PMID: 19559622 Review. - Interactions of MAP/microtubule affinity regulating kinases with the adaptor complex AP-2 of clathrin-coated vesicles.
Schmitt-Ulms G, Matenia D, Drewes G, Mandelkow EM. Schmitt-Ulms G, et al. Cell Motil Cytoskeleton. 2009 Aug;66(8):661-72. doi: 10.1002/cm.20394. Cell Motil Cytoskeleton. 2009. PMID: 19536824 - Structure and function of polarity-inducing kinase family MARK/Par-1 within the branch of AMPK/Snf1-related kinases.
Marx A, Nugoor C, Panneerselvam S, Mandelkow E. Marx A, et al. FASEB J. 2010 Jun;24(6):1637-48. doi: 10.1096/fj.09-148064. Epub 2010 Jan 13. FASEB J. 2010. PMID: 20071654 Review.
Cited by
- AMPKα-like proteins as LKB1 downstream targets in cell physiology and cancer.
Molina E, Hong L, Chefetz I. Molina E, et al. J Mol Med (Berl). 2021 May;99(5):651-662. doi: 10.1007/s00109-021-02040-y. Epub 2021 Mar 4. J Mol Med (Berl). 2021. PMID: 33661342 Review. - Microglial TNFα orchestrates protein phosphorylation in the cortex during the sleep period and controls homeostatic sleep.
Pinto MJ, Cottin L, Dingli F, Laigle V, Ribeiro LF, Triller A, Henderson F, Loew D, Fabre V, Bessis A. Pinto MJ, et al. EMBO J. 2023 Jan 4;42(1):e111485. doi: 10.15252/embj.2022111485. Epub 2022 Nov 16. EMBO J. 2023. PMID: 36385434 Free PMC article. - Prostate-derived sterile 20-like kinases (PSKs/TAOKs) phosphorylate tau protein and are activated in tangle-bearing neurons in Alzheimer disease.
Tavares IA, Touma D, Lynham S, Troakes C, Schober M, Causevic M, Garg R, Noble W, Killick R, Bodi I, Hanger DP, Morris JD. Tavares IA, et al. J Biol Chem. 2013 May 24;288(21):15418-29. doi: 10.1074/jbc.M112.448183. Epub 2013 Apr 12. J Biol Chem. 2013. PMID: 23585562 Free PMC article. - Tao controls epithelial morphogenesis by promoting Fasciclin 2 endocytosis.
Gomez JM, Wang Y, Riechmann V. Gomez JM, et al. J Cell Biol. 2012 Dec 24;199(7):1131-43. doi: 10.1083/jcb.201207150. J Cell Biol. 2012. PMID: 23266957 Free PMC article. - Solution structure of the kinase-associated domain 1 of mouse microtubule-associated protein/microtubule affinity-regulating kinase 3.
Tochio N, Koshiba S, Kobayashi N, Inoue M, Yabuki T, Aoki M, Seki E, Matsuda T, Tomo Y, Motoda Y, Kobayashi A, Tanaka A, Hayashizaki Y, Terada T, Shirouzu M, Kigawa T, Yokoyama S. Tochio N, et al. Protein Sci. 2006 Nov;15(11):2534-43. doi: 10.1110/ps.062391106. Protein Sci. 2006. PMID: 17075132 Free PMC article.
References
- Anostario M., Harrison,M.L. and Geahlen,R.L. (1990) Immunochemical detection of adenine nucleotide-binding proteins with antibodies to 5′-p-fluorosulfonylbenzoyladenosine. Analyt. Biochem., 190, 60–65. - PubMed
- Benton R., Palacios,I. and St Johnston,D. (2002) Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev. Cell, 3, 659–671. - PubMed
- Biernat J., Gustke,N., Drewes,G., Mandelkow,E.-M. and Mandelkow,E. (1993) Phosphorylation of Ser 262 strongly reduces the binding of tau protein to microtubules. Neuron, 11, 153–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases