Dysfunctional MreB inhibits chromosome segregation in Escherichia coli - PubMed (original) (raw)
Dysfunctional MreB inhibits chromosome segregation in Escherichia coli
Thomas Kruse et al. EMBO J. 2003.
Abstract
The mechanism of prokaryotic chromosome segregation is not known. MreB, an actin homolog, is a shape-determining factor in rod-shaped prokaryotic cells. Using immunofluorescence microscopy we found that MreB of Escherichia coli formed helical filaments located beneath the cell surface. Flow cytometric and cytological analyses indicated that MreB-depleted cells segregated their chromosomes in pairs, consistent with chromosome cohesion. Overexpression of wild-type MreB inhibited cell division but did not perturb chromosome segregation. Overexpression of mutant forms of MreB inhibited cell division, caused abnormal MreB filament morphology and induced severe localization defects of the nucleoid and of the oriC and terC chromosomal regions. The chromosomal terminus regions appeared cohered in both MreB-depleted cells and in cells overexpressing mutant forms of MreB. Our observations indicate that MreB filaments participate in directional chromosome movement and segregation.
Figures
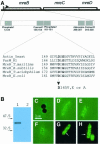
Fig. 1. Subcellular localization of the MreB protein in E.coli. (A) Schematic drawing of the mreBCD locus of E.coli. The mreB gene (347 codons) is shown as a hatched box, mreC (367 codons) and mreD (162 codons) as open boxes. The broken arrow pointing right-wards indicates the mreBCD promoter. The first enlargement shows the five regions in MreB that exhibit similarity to analogous regions present in all proteins with the actin fold (Bork et al., 1992). Numbers are amino acids in MreB. The second enlargement shows an alignment of the phosphate-2 regions of actin (from yeast), ParM from plasmid R1, MreBs from T.maritima (a deep branching eubacterium), B.subtilis, Thermoplasma acidophilum (an archaeon) and E.coli. Conserved amino acids are in bold. Amino acid changes at position +165 in the phosphate-2 region of MreB of E.coli are indicated. (B) Immunoblot showing the specificity of the affinity purified anti-MreB antibodies. Lane 1, protein extract from wild-type cells (MC1000); lane 2, MC1000Δ_mreB_. Molecular weight markers are shown to the left. (C–H) Subcellular localization of MreB visualized by combined phase-contrast and immunofluorescence microscopy using affinity purified anti-MreB antibodies. (C) Cells of MC1000Δ_mreB_; (D–F) cells of MC1000; (G and H) cells of MC1000 visualized by fluorescence microscopy in the absence of phase-contrast. Arrows point to helical structures. Cells were grown at 30°C in AB glycerol medium supplemented with 0.025% casamino acids.
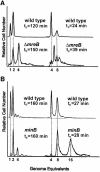
Fig. 2. Number of replication origins in single cells counted by flow cytometry. (A) Shown are flow cytometric histograms of E.coli strains MC1000 (wild type) and MC1000Δ_mreB_ obtained after 4 h of treatment with rifampicin. Rifampicin was added to inhibit new replication initiations at oriC and to allow for run-out synthesis of ongoing rounds of DNA replication. DNA was stained with ethidium bromide. Left, cells were grown in AB glycerol minimal medium at 37°C. Right, the cells were grown in LB medium. The cell mass doubling times at the times of addition of rifampicin are indicated. (B) Flow-cytometric histograms of PB103 (wild type) and PB114 (minB) cells grown as described above.
Fig. 3. Subcellular localization of oriC and terC. oriC and terC regions were visualized by Gfp–ParB nucleation on parS sites inserted in bglF (near oriC) or in relBE (near terC). All strains shown contained plasmid pTK536 that expressed a Gfp–ParB fusion protein. Cells were grown at 37°C in AB glycerol or LB medium with a doubling time of 145 or 27 min (wild type) and 174 or 44 min (Δ_mreB_), respectively. (A) Expression of Gfp–ParB was induced with 0.2% arabinose 180 min before inspection. (a and b) MC1000 with parS inserted near oriC grown in AB glycerol. (c and d) Same strain grown in LB medium. (e–g) MC1000 with parS inserted near terC grown in AB glycerol. (h) Same strain grown in LB medium. (B) MC1000Δ_mreB_ cells with parS near oriC (a–f) or terC (g–l) grown in AB glycerol. (C) MC1000Δ_mreB_ cells with parS inserted at oriC (a–c) or terC (d–f) grown in LB medium.
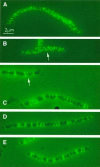
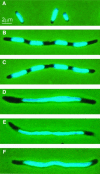
Fig. 4. MreB localization in elongated cells visualized by immuno-fluorescence microscopy. Subcellular localization of MreB in wild-type cells treated with cephalexin or in wild-type cells expressing native or mutated forms of mreB present in the low-copy-number expression vector pNDM220. Cells were grown at 30°C in AB glycerol medium supplemented with 0.025% casamino acids. Cells were induced with 100 µM of IPTG for five mass doublings. Localization of MreB was performed using affinity purified anti-MreB antibodies and combined phase-contrast and immunofluorescence microscopy. Arrows point to unmistakable helical structures. (A) Cells of MC1000 (mreBCD+) were treated with 10 µg/ml cephalexin for three mass doublings prior to microscopy. (B) MC1000/pTK505 (wild-type mreB); (C) MC1000/pTK509 (mreB165V); (D) MC1000/pTK510 (mreB165E); (E) MC1000/pTK511 (mreB165A).
Fig. 5. Inhibition of nucleoid segregation by mutant forms of MreB. Nucleoid distribution in cells of MC1000 (mreBCD+) treated with cephalexin or MC1000 expressing native or mutated forms of mreB present in the expression vector pNDM220. Cells were grown at 30°C in AB glycerol medium supplemented with 0.025% casamino acids and stained with DAPI. MreB synthesis was induced with IPTG (100 µM) for five mass doublings. (A) MC1000 wild-type cells grown exponentially; (B) MC1000 wild-type cells were treated with 10 µg/ml cephalexin for three mass doublings prior to microscopy; (C) cells of MC1000/pTK505 (wild-type mreB); (D) MC1000/pTK509 (mreB165V); (E) MC1000/pTK510 (mreB165E); (F) MC1000/pTK511 (mreB165A).
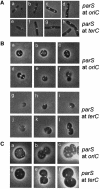
Fig. 6. Defect localization of oriC and terC in cells expressing mutant forms of MreB. Localization of oriC and terC by Gfp–ParB labeling in wild-type cells treated with cephalexin or in wild-type cells expressing native or mutated forms of mreB present in expression vector pNDM220. Cells were grown at 30°C in AB glycerol medium supplemented with 0.025% casamino acids. MreB synthesis was induced with IPTG (100 µM) for five mass doublings and Gfp–ParB synthesis (from pTK536) with 0.2% arabinose for 180 min. (A–E) Cells with parS inserted into bglF (near oriC); (F–J) cells with parS inserted into relBE (near terC). (A) MC1000_bglF_::parS/pTK536 cells treated with 10 µg/ml cephalexin for three mass doublings; (B) MC1000_bglF_::parS/pTK536/pTK505 (wild-type mreB); (C) MC1000_bglF_::parS/pTK536/pTK509 (mreB165V); (D) MC1000_bglF_::parS/pTK536/pTK510 (mreB165E); (E) MC1000_bglF_::parS/pTK536/pTK511 (mreB165A); (F) MC1000_relBE_:: parS/pTK536 treated with 10 µg/ml cephalexin; (G) MC1000_relBE_:: parS/pTK536/pTK505 (wild-type mreB); (H) MC1000_relBE_::parS/pTK536/pTK509 (mreB165V); (I) MC1000_relBE_::parS/pTK536/pTK510 (mreB165E); (J) MC1000_relBE_::parS/pTK536/pTK511 (mreB165A).
Fig. 7. Pairing explains the chromosome segregation pattern in cells lacking MreB. Chromosomes stay paired in Δ_mreB_ cells (cohesion). The model assumes that replication allows separation of the old chromosomes and that the newly replicated sister chromosomes stay together as pairs. Pairing explains the even number of chromosomes in the Δ_mreB_ strain seen at all growth rates. During division of Δ_mreB_ cells, paired chromosomes segregate randomly, thus leading to anucleate cells and to cells with an even number of chromosomes. The lack of coordination of chromosome segregation also explains the broad copy-number distribution of chromosomes seen in the Δ_mreB_ cells. The schematic does not show all possible combinations of the number of chromosomes in rapidly growing Δ_mreB_ cells. Symbols: chromosomes are shown as large circles, origins of replication as closed dots and termini as open dots. For simplicity, chromosomes are shown as full circles and the figure does not take ongoing replication into account.
Similar articles
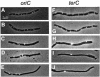
- DNA and origin region segregation are not affected by the transition from rod to sphere after inhibition of Escherichia coli MreB by A22.
Karczmarek A, Martínez-Arteaga R, Alexeeva S, Hansen FG, Vicente M, Nanninga N, den Blaauwen T. Karczmarek A, et al. Mol Microbiol. 2007 Jul;65(1):51-63. doi: 10.1111/j.1365-2958.2007.05777.x. Mol Microbiol. 2007. PMID: 17581120 - Actin homolog MreB and RNA polymerase interact and are both required for chromosome segregation in Escherichia coli.
Kruse T, Blagoev B, Løbner-Olesen A, Wachi M, Sasaki K, Iwai N, Mann M, Gerdes K. Kruse T, et al. Genes Dev. 2006 Jan 1;20(1):113-24. doi: 10.1101/gad.366606. Genes Dev. 2006. PMID: 16391237 Free PMC article. - MreB is important for cell shape but not for chromosome segregation of the filamentous cyanobacterium Anabaena sp. PCC 7120.
Hu B, Yang G, Zhao W, Zhang Y, Zhao J. Hu B, et al. Mol Microbiol. 2007 Mar;63(6):1640-52. doi: 10.1111/j.1365-2958.2007.05618.x. Mol Microbiol. 2007. PMID: 17367385 - Structural and physical aspects of bacterial chromosome segregation.
Woldringh CL, Nanninga N. Woldringh CL, et al. J Struct Biol. 2006 Nov;156(2):273-83. doi: 10.1016/j.jsb.2006.04.013. Epub 2006 May 20. J Struct Biol. 2006. PMID: 16828313 Review. - Bacterial DNA segregation by the actin-like MreB protein.
Kruse T, Gerdes K. Kruse T, et al. Trends Cell Biol. 2005 Jul;15(7):343-5. doi: 10.1016/j.tcb.2005.05.002. Trends Cell Biol. 2005. PMID: 15922599 Review.
Cited by
- Multidimensional view of the bacterial cytoskeleton.
Celler K, Koning RI, Koster AJ, van Wezel GP. Celler K, et al. J Bacteriol. 2013 Apr;195(8):1627-36. doi: 10.1128/JB.02194-12. Epub 2013 Feb 15. J Bacteriol. 2013. PMID: 23417493 Free PMC article. Review. - Coarse-grained simulations of bacterial cell wall growth reveal that local coordination alone can be sufficient to maintain rod shape.
Nguyen LT, Gumbart JC, Beeby M, Jensen GJ. Nguyen LT, et al. Proc Natl Acad Sci U S A. 2015 Jul 14;112(28):E3689-98. doi: 10.1073/pnas.1504281112. Epub 2015 Jun 30. Proc Natl Acad Sci U S A. 2015. PMID: 26130803 Free PMC article. - Manipulating each MreB of Bdellovibrio bacteriovorus gives diverse morphological and predatory phenotypes.
Fenton AK, Lambert C, Wagstaff PC, Sockett RE. Fenton AK, et al. J Bacteriol. 2010 Mar;192(5):1299-311. doi: 10.1128/JB.01157-09. Epub 2009 Dec 18. J Bacteriol. 2010. PMID: 20023029 Free PMC article. - Effects of polymerization and nucleotide identity on the conformational dynamics of the bacterial actin homolog MreB.
Colavin A, Hsin J, Huang KC. Colavin A, et al. Proc Natl Acad Sci U S A. 2014 Mar 4;111(9):3585-90. doi: 10.1073/pnas.1317061111. Epub 2014 Feb 18. Proc Natl Acad Sci U S A. 2014. PMID: 24550504 Free PMC article. - Effect of A22 on the Conformation of Bacterial Actin MreB.
Awuni E, Mu Y. Awuni E, et al. Int J Mol Sci. 2019 Mar 15;20(6):1304. doi: 10.3390/ijms20061304. Int J Mol Sci. 2019. PMID: 30875875 Free PMC article.
References
- Aussel L., Barre,F.X., Aroyo,M., Stasiak,A., Stasiak,A.Z. and Sherratt,D. (2002) FtsK is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell, 108, 195–205. - PubMed
- Bernander R. and Nordstrom,K. (1990) Chromosome replication does not trigger cell division in E. coli. Cell, 60, 365–374. - PubMed
- Carballido-Lopez R. and Errington,J. (2003) The bacterial cytoskeleton: in vivo dynamics of the actin-like protein Mbl of Bacillus subtilis. Dev. Cell, 4, 19–28. - PubMed
- Casadaban M.J. and Cohen,S.N. (1980) Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol., 138, 179–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases