Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex - PubMed (original) (raw)
Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex
Jost Enninga et al. Mol Cell Biol. 2003 Oct.
Abstract
Sec13 is a constituent of the endoplasmic reticulum and the nuclear pore complex (NPC). At the endoplasmic reticulum, Sec13 is involved in the biogenesis of COPII-coated vesicles, whereas at the NPC its function is unknown. We show here, by yeast two-hybrid screenings and biochemical assays, that a region at the amino terminus of the human nuclear pore complex protein Nup96 interacts with the WD (Trp-Asp) repeat region of human Sec13. By using immunofluorescence and confocal and immunoelectron microscopy, we found that in interphase, Sec13 and Nup96 are localized at both sides of the NPC in addition to other intracellular sites. In mitosis, Sec13 was found dispersed throughout the cell, whereas a pool of Nup96 colocalized with the spindle apparatus. Photobleaching experiments showed that Sec13 shuttles between intranuclear sites and the cytoplasm, and a fraction of Sec13 is stably associated with NPCs. Cotransfection of Sec13 and the Sec13 binding site of Nup96 decreased the mobile pool of Sec13, demonstrating the interaction of Sec13 and Nup96 in vivo. Targeting studies showed that Sec13 is actively transported into the nucleus and contains a nuclear localization signal. These results indicate that Sec13 stably interacts with Nup96 at the NPC during interphase and that the shuttling of Sec13 between the nucleus and the cytoplasm may couple and regulate functions between these two compartments.
Figures
FIG. 1.
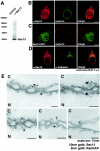
Interaction of Sec13 with an amino-terminal region of Nup96 in yeast two-hybrid screenings performed with multiple libraries. (A) β-Galactosidase assays demonstrate interaction between Sec13 cDNAs (Sec13_12, Sec13_13, Sec13_20, Sec13_21, and Sec13_28) isolated independently in yeast two-hybrid screenings and Nup96 (amino acids 1 to 378) as bait. Baits include the empty vector (pBUTE) and the following proteins cloned in frame with the GAL4 DNA binding domain: Nup96 N terminus (amino acids 1 to 378), the EF hand domain of intersectin (INT EH), the coiled-coiled domain of intersectin (INT COIL), and the human SKP1 homologue (F19). Preys include the empty prey (activation domain) vector (pGADC1), Sec13 cDNAs isolated independently from different screenings, mouse epsin, and human Fbox5. β-Galactosidase activity was assayed in cultures expressing the bait and prey following mating and selection in interaction selection medium. Purple indicates a positive signal; yellow is negative. (B) The table shows baits and preys as in A. Growth was measured by optical density at 620 nm readings 48 h after dilution of mating mixtures into interaction selection medium. (C) β-Galactosidase readings from A. High optical density values marked in yellow represent positive interactions. (D and E) Wild-type Nup98 and Nup96 and truncated mutants of Nup96 were transcribed and translated in vitro in the presence of [35S]methionine and incubated with immobilized recombinant GST-Sec13 (1 μg) as described in Materials and Methods. Bound and unbound fractions were analyzed by SDS-PAGE and autoradiography. (F) Schematic representation of wild-type and truncated mutants of Nup96. Shown in red is the 6-kDa region that is present in all isoforms of the Nup98-Nup96 gene. Shown in purple is the 9-kDa region unique to the Nup96 isoform. (G) Wild-type Nup96 and truncated mutants of Nup96 were transcribed and translated in vitro in the presence of [35S]methionine and incubated with 1 μg of immobilized recombinant GST-Sec13 mutants (amino acids 1 to 302 and 301 to 322) as described in Materials and Methods. Bound and unbound fractions were analyzed by SDS-PAGE and autoradiography.
FIG. 2.
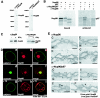
Immunolocalization of Sec13 to both sides of the NPC in addition to cytoplasmic and intranuclear sites. (A) Immunoblot analysis of HeLa cell lysates performed with anti-Sec13 antibodies and probed with either preimmune serum (control, lane 1) or antibodies against Sec13 (lane 2). (B) Immunofluorescence and confocal microscopy of HeLa cells, which were fixed, permeabilized with saponin, and labeled with anti-Sec13 antibodies and mAb414 (labels some FG repeat-containing Nups). (C) HeLa cells transfected with GFP-Sec13 and analyzed by confocal microscopy show staining similar to the observed labeling of endogenous Sec13 shown in B and D. (D) HeLa cells were processed as in B except that anticalnexin antibodies were used. Endoplasmic reticulum labeling is observed. The merged image shows partial colocalization of Sec13 with calnexin at the endoplasmic reticulum. (E) Isolated rat liver nuclear envelopes were probed with rabbit anti-Sec13 antibodies and mouse anti-RanGAP1 monoclonal antibody 19C7. Rabbit and mouse antibodies were detected with 10-nm and 5-nm gold-coupled secondary antibodies, respectively. Envelopes were processed for thin sectioning and observed by electron microscopy. C, cytoplasmic side; N, nucleoplasmic side.
FIG. 3.
Alternatively spliced forms of the Nup98-Nup96 gene. (A) Immunoblot analysis of cell extracts obtained from U937 cells performed with preimmune serum (control), anti-Nup96 antibodies, or anti-Nup96/p87 antibodies. (B) Differential recognition of Nup96 and p87 by the anti-Nup96 and anti-Nup96/p87 antibodies. The anti-Nup96 antibodies were developed against the 9-kDa polypeptide region which is unique to the Nup96 isoform (amino acids 1485 to 1559), and the anti-Nup96/p87 antibodies were developed against a region which is common to Nup96 and p87 (amino acids 1291 to 1482). Nup96 and p87 were in vitro transcribed and translated in a reticulocyte lysate system and immunoprecipitated with the indicated antibodies to determine specificity. (C) Immunoblot analysis of cell extracts obtained from U937 cells either treated or untreated with interferon gamma performed with anti-Nup96 antibodies or anti-Nup96/p87 antibodies. Nup96 is expressed in higher amounts compared to the p87 isoform. (D) Nup96 is localized at the NPC, intranuclear sites, and nucleolus. (a to f) U937 cells were first fixed with formaldehyde, permeabilized with Triton X-100, and immunolabeled with anti-Nup96 and mAb414 antibodies (a to c) or anti-Nup96 and antinucleolin antibodies (d to f). (g to i) U937 cells were treated with Triton X-100 prior to fixation and labeled with anti-Nup96 and mAb414 antibodies. (E) Immunolocalization of Nup96 to both the cytoplasmic and nucleoplasmic sides of the NPC. Isolated rat liver nuclear envelopes were probed with rabbit anti-Nup96/p87 or anti-Nup96 antibodies and mouse anti-RanGAP1 monoclonal antibody 19C7. Rabbit and mouse antibodies were detected with 10-nm and 5-nm gold-coupled secondary antibodies, respectively. Envelopes were processed for thin sectioning and observed by electron microscopy. C, cytoplasmic side; N, nucleoplasmic side.
FIG. 4.
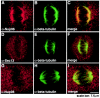
Nup96 colocalized with the spindle apparatus during mitosis, whereas Sec13 and Nup98 were distributed throughout the cell. Immunofluorescence and confocal microscopy were performed with HeLa cells at mitosis. Cells were fixed with formaldehyde, permeabilized with Triton X-100, and labeled with anti-Nup96 and anti-β-tubulin antibodies (A to C), with anti-Sec13 and anti-β-tubulin antibodies (D to F), or with anti-Nup98 and anti-β-tubulin antibodies (G to I). Merged images showed partial colocalization of Nup96 and β-tubulin at the spindle apparatus, whereas Sec13 and Nup98 did not colocalize with the spindles and was dispersed throughout the cell.
FIG. 5.
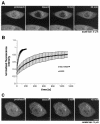
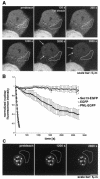
Dynamics of Sec13 in the nucleus and in the cytoplasm. (A) FRAP analysis of HeLa cells transfected with Sec13-GFP. The nucleus was bleached, and the fluorescence recovery in the nucleus was monitored. The image shows a confocal section at the equatorial plane of the nucleus. Images were acquired every 15 s. (B) Plots show fluorescence recovery of nuclear Sec13-GFP in the bleached area. Measurements of fluorescence intensity were subtracted from background fluorescence and normalized from loss of fluorescence during bleaching and imaging. Error bars are standard deviations (n = 3). (C) FRAP analysis of HeLa cells transfected with Sec13-GFP where the NPC, endoplasmic reticulum, and cytosol were bleached and fluorescence recovery was followed. Note the instant fluorescence recovery at the endoplasmic reticulum after 5 min as opposed to the NPC, which had still not recovered after 45 min.
FIG. 6.
Shuttling of Sec13 between the nucleus and the cytoplasm. (A) FLIP experiments in HeLa cells transfected with Sec13-GFP. The bleached region highlighted includes the endoplasmic reticulum and cytosol. Photobleaching was performed every 4 min, and images were scanned every 30 s. Note the instant recovery of fluorescence at the endoplasmic reticulum after bleaching in contrast to the sequential loss of fluorescence observed inside the nucleus and not at the NPC. Arrows show NPC labeling, which is better visualized in the last panels. (B) Plot of fluorescence loss in the nucleus over time in cells transfected with Sec13-GFP, EGFP alone, or PML-EGFP. Error bars are standard deviations (n = 3). (C) FLIP experiments in HeLa cells transfected with PML-EGFP performed as in A. Fluorescence loss in the nucleus was not observed after photobleaching the cytoplasm 10 times.
FIG. 7.
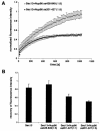
Interference with Sec13 dynamics by the Sec13-binding site of Nup96. (A) Plots show FRAP assays performed with HeLa cells cotransfected with Sec13-GFP and Nup96 (amino acids 201 to 427; the Sec13 binding site of Nup96), or Sec13-GFP and Nup96 (amino acids 428 to 849, which does not interact with Sec13). Measurements were performed as in Fig. 5 (n = 6). (B) Histograms show measurements by FRAP assays as in A of the total number of mobile Sec13-GFP molecules in the absence or presence of the Sec13-binding site of Nup96 at ratios of 1:1 or 1:5 or the carboxyl-terminal region of Nup96 (amino acids 428 to 849) at a ratio of 1:5.
FIG. 8.
The carboxy terminus of Sec13 contains a nuclear localization signal(s). HeLa cells were transfected with Myc-pyruvate kinase alone (A), Myc-pyruvate kinase fused with full-length Sec13 (amino acids 1 to 322) (B), or Myc-pyruvate kinase fused with different regions of Sec13 (amino acids 1 to 243; 244 to 322; 1 to 302; and 301 to 322) (C to F). Transiently expressed proteins were detected by immunofluorescence and confocal microscopy with anti-Myc (9E10) and anti-Nup358 antibodies, which were labeled with secondary antibodies conjugated with indocarbocyanine and fluorescein isothiocyanate, respectively. (G) Schematic representation of the constructs.
Similar articles
- Nup98 localizes to both nuclear and cytoplasmic sides of the nuclear pore and binds to two distinct nucleoporin subcomplexes.
Griffis ER, Xu S, Powers MA. Griffis ER, et al. Mol Biol Cell. 2003 Feb;14(2):600-10. doi: 10.1091/mbc.e02-09-0582. Mol Biol Cell. 2003. PMID: 12589057 Free PMC article. - Depletion of a single nucleoporin, Nup107, prevents the assembly of a subset of nucleoporins into the nuclear pore complex.
Boehmer T, Enninga J, Dales S, Blobel G, Zhong H. Boehmer T, et al. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):981-5. doi: 10.1073/pnas.252749899. Epub 2003 Jan 27. Proc Natl Acad Sci U S A. 2003. PMID: 12552102 Free PMC article. - A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186-kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96.
Fontoura BM, Blobel G, Matunis MJ. Fontoura BM, et al. J Cell Biol. 1999 Mar 22;144(6):1097-112. doi: 10.1083/jcb.144.6.1097. J Cell Biol. 1999. PMID: 10087256 Free PMC article. - Nuclear pore complex biogenesis.
Fernandez-Martinez J, Rout MP. Fernandez-Martinez J, et al. Curr Opin Cell Biol. 2009 Aug;21(4):603-12. doi: 10.1016/j.ceb.2009.05.001. Epub 2009 Jun 11. Curr Opin Cell Biol. 2009. PMID: 19524430 Free PMC article. Review. - Making COPII coats.
Kirchhausen T. Kirchhausen T. Cell. 2007 Jun 29;129(7):1251-2. doi: 10.1016/j.cell.2007.06.015. Cell. 2007. PMID: 17604713 Review.
Cited by
- The Arabidopsis SUPPRESSOR OF AUXIN RESISTANCE proteins are nucleoporins with an important role in hormone signaling and development.
Parry G, Ward S, Cernac A, Dharmasiri S, Estelle M. Parry G, et al. Plant Cell. 2006 Jul;18(7):1590-603. doi: 10.1105/tpc.106.041566. Epub 2006 Jun 2. Plant Cell. 2006. PMID: 16751346 Free PMC article. - Structural and functional studies of Nup107/Nup133 interaction and its implications for the architecture of the nuclear pore complex.
Boehmer T, Jeudy S, Berke IC, Schwartz TU. Boehmer T, et al. Mol Cell. 2008 Jun 20;30(6):721-31. doi: 10.1016/j.molcel.2008.04.022. Mol Cell. 2008. PMID: 18570875 Free PMC article. - Nucleoporin levels regulate cell cycle progression and phase-specific gene expression.
Chakraborty P, Wang Y, Wei JH, van Deursen J, Yu H, Malureanu L, Dasso M, Forbes DJ, Levy DE, Seemann J, Fontoura BM. Chakraborty P, et al. Dev Cell. 2008 Nov;15(5):657-67. doi: 10.1016/j.devcel.2008.08.020. Dev Cell. 2008. PMID: 19000832 Free PMC article. - COPII cage assembly factor Sec13 integrates information flow regulating endomembrane function in response to human variation.
Anglès F, Gupta V, Wang C, Balch WE. Anglès F, et al. Sci Rep. 2024 May 3;14(1):10160. doi: 10.1038/s41598-024-60687-2. Sci Rep. 2024. PMID: 38698045 Free PMC article. - Sec24C is an HIV-1 host dependency factor crucial for virus replication.
Rebensburg SV, Wei G, Larue RC, Lindenberger J, Francis AC, Annamalai AS, Morrison J, Shkriabai N, Huang SW, KewalRamani V, Poeschla EM, Melikyan GB, Kvaratskhelia M. Rebensburg SV, et al. Nat Microbiol. 2021 Apr;6(4):435-444. doi: 10.1038/s41564-021-00868-1. Epub 2021 Mar 1. Nat Microbiol. 2021. PMID: 33649557 Free PMC article.
References
- Belgareh, N., G. Rabut, S. W. Bai, M. van Overbeek, J. Beaudouin, N. Daigle, O. V. Zatsepina, F. Pasteau, V. Labas, M. Fromont-Racine, J. Ellenberg, and V. Doye. 2001. An evolutionary conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J. Cell Biol. 154:1147-1160. - PMC - PubMed
- Carmo-Fonseca, M. 2002. The contribution of nuclear compartmentalization to gene regulation. Cell 108:513-521. - PubMed
- Combet, C., C. Blanchet, C. Geourjon, and G. Deleage. 200. NPS@Network Protein Sequence Analysis. Trends Biochem. Sci. 25:147-150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous