SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system - PubMed (original) (raw)
SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system
Barbara J Schiemann et al. Mol Biol Cell. 2003 Oct.
Abstract
Secreted protein, acidic and rich in cysteine (SPARC) is a multifunctional secreted protein that regulates cell-cell and cell-matrix interactions, leading to alterations in cell adhesion, motility, and proliferation. Although SPARC is expressed in epithelial cells, its ability to regulate epithelial cell growth remains largely unknown. We show herein that SPARC strongly inhibited DNA synthesis in transforming growth factor (TGF)-beta-sensitive Mv1Lu cells, whereas moderately inhibiting that in TGF-beta-insensitive Mv1Lu cells (i.e., R1B cells). Overexpression of dominant-negative Smad3 in Mv1Lu cells, which abrogated growth arrest by TGF-beta, also attenuated growth arrest stimulated by SPARC. Moreover, the extracellular calcium-binding domain of SPARC (i.e., SPARC-EC) was sufficient to inhibit Mv1Lu cell proliferation but not that of R1B cells. Similar to TGF-beta and thrombospondin-1, treatment of Mv1Lu cells with SPARC or SPARC-EC stimulated Smad2 phosphorylation and Smad2/3 nuclear translocation: the latter response to all agonists was abrogated in R1B cells or by pretreatment of Mv1Lu cells with neutralizing TGF-beta antibodies. SPARC also stimulated Smad2 phosphorylation in MB114 endothelial cells but had no effect on bone morphogenetic protein-regulated Smad1 phosphorylation in either Mv1Lu or MB114 cells. Finally, SPARC and SPARC-EC stimulated TGF-beta-responsive reporter gene expression through a TGF-beta receptor- and Smad2/3-dependent pathway in Mv1Lu cells. Collectively, our findings identify a novel mechanism whereby SPARC inhibits epithelial cell proliferation by selectively commandeering the TGF-beta signaling system, doing so through coupling of SPARC-EC to a TGF-beta receptor- and Smad2/3-dependent pathway.
Figures
Figure 1.
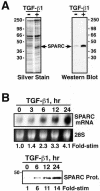
TGF-β induces SPARC expression in Mv1Lu cells. (A) Metabolically labeled naive- or TGF-β–conditioned media were collected and concentrated by trichloroacetic acid/deoxycholate precipitation. Duplicate samples were fractionated through 10% SDS-PAGE before visualization of SPARC by silver staining (left) and Western blotting with monoclonal anti-SPARC antibodies (right). (B) Total RNA (10 μg/lane) prepared from TGF-β1–treated Mv1Lu cells was hybridized with a radiolabeled human SPARC cDNA probe. The uniformity of mRNA loading was monitored by ethidium bromide staining to visualize the 28S rRNA (top). Naïve and TGF-β–conditioned media were collected and concentrated by trichloroacetic acid/deoxycholate precipitation before fractionation through 10% SDS-PAGE. SPARC was visualized by Western blotting with monoclonal anti-SPARC antibodies as described above (bottom).
Figure 2.
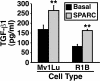
SPARC induces TGF-β1 expression in Mv1Lu cells. Naïve and SPARC-conditioned Mv1Lu and R1B cell media were collected and acidified to activate total TGF-β. After sample neutralization, TGF-β1 concentrations were determined by ELISA analysis. Data are the mean TGF-β1 concentrations (± SEM) of two independent experiments.
Figure 3.
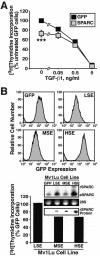
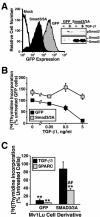
Constitutive SPARC expression inhibits Mv1Lu cell proliferation. (A) GFP- or SPARC-expressing Mv1Lu cells were incubated with increasing concentrations of TGF-β1 (0–5 ng/ml) for 48 h. Cellular DNA was radiolabeled with [3H]thymidine and quantitated by scintillation counting. Data are the mean (± SEM) of three independent experiments presented as the percentage of [3H]thymidine incorporation normalized to untreated GFP-expressing cells. SPARC expression significantly decreased DNA synthesis in Mv1Lu cells (***p < 0.05; Student's t test). (B) SPARC-infected Mv1Lu cells were FACS-sorted by GFP expression to obtain stable polyclonal populations of low (LSE), medium (MSE), or high (HSE) SPARC-expressing cells (top), which then were compared for rates of DNA synthesis by [3H]thymidine assay. Inset shows expression of recombinant (rSPARC) and endogenous (eSPARC) SPARC mRNA by sorted individual Mv1Lu cell populations as detected by Northern blotting. Also shown is the expression of rSPARC captured from conditioned media of individual Mv1Lu cell populations and visualized by anti-Myc immunoblotting. Shown is a representative experiment that was repeated twice with similar results.
Figure 4.
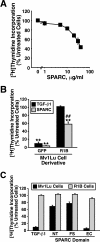
Purified SPARC and recombinant SPARC-EC inhibit Mv1Lu cell proliferation in part through stimulation of the TGF-β–signaling system. (A) Mv1Lu cells were incubated with increasing concentrations of purified bovine SPARC (0–40 μg/ml) as indicated. Changes in DNA synthesis rates were quantified by a [3H]thymidine assay. Data are the mean (± SEM) of two to five independent experiments presented as the percentage of [3H]thymidine incorporation normalized to untreated cells. Unless shown, all error bars are within individual symbol. (B) GFP-expressing Mv1Lu or R1B cells were treated with TGF-β1 (5 ng/ml) or purified bovine SPARC (30 μg/ml) for 48 h before addition of [3H]thymidine to label cellular DNA. Data are the mean (± SEM) of three independent experiments presented as the percentage of [3H]thymidine incorporation normalized to appropriate untreated cells. SPARC significantly decreased DNA synthesis in treated cells (**p < 0.05; Student's t test). The decrease in DNA synthesis stimulated by SPARC was significantly attenuated as compared with Mv1Lu cells (##p < 0.05; Student's t test). (C) Mv1Lu or R1B cells were stimulated with TGF-β (5 ng/ml), recombinant SPARC-NT (30 μg/ml), recombinant SPARC-FS (30 μg/ml), or recombinant SPARC-EC (30 μg/ml) as indicated. Cellular DNA was radiolabeled with [3H]thymidine and quantified by scintillation counting. Data are the mean (± SEM) of three independent experiments presented as the percentage of [3H]thymidine incorporation normalized to appropriate untreated cells.
Figure 5.
Dominant-negative Smad3/3A expression reduces the antiproliferative activities of SPARC on Mv1Lu cells. (A) GFP- or dominant-negative Smad3/3A-expressing Mv1Lu cells were isolated by FACS-sorting for GFP expression. Expression of Smad3/3A insignificantly reduced Smad2 phosphorylation stimulated by TGF-β1 (inset). (B) GFP- or Smad3/3A-expressing Mv1Lu cells were incubated in the absence or presence of increasing concentrations of TGF-β1 as indicated. Cellular DNA was radiolabeled with [3H]thymidine and quantitated by scintillation counting. Data are the mean (± SEM) of two independent experiments presented as the percentage of [3H]thymidine incorporation normalized to untreated GFP-expressing cells. (C) GFP- or Smad3/3A-expressing Mv1Lu cells were stimulated with TGF-β1 (5 ng/ml) or purified bovine SPARC (30 μg/ml) as indicated. Data are the mean (± SEM) of three independent experiments presented as the percentage of [3H]thymidine incorporation normalized to appropriate untreated cells. SPARC significantly decreased DNA synthesis in treated cells (**p < 0.05; Student's t test). The decrease in DNA synthesis stimulated by SPARC was significantly attenuated compared with Mv1Lu cells (##p < 0.05; Student's t test).
Figure 6.
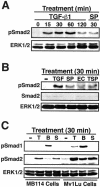
SPARC and SPARC-EC stimulate Smad2/3 nuclear translocation in Mv1Lu Cells. (A) Mv1Lu or R1B cells were incubated in the absence or presence of neutralizing TGF-β antibodies (5 μg/ml) for 60 min before stimulation with TGF-β1 (2.5 ng/ml), purified bovine SPARC (30 μg/ml), recombinant SPARC-EC (30 μg/ml), or thrombospondin-1 (30 μg/ml) for an additional 30 min at 37°C. Afterward, the cells were fixed in 4% paraformaldehyde and processed for indirect immunofluorescence with anti-Smad2/3 antibodies as described under MATERIALS AND METHODS. Images were captured on a Diaphot microscope (40× magnification). Data are from a representative experiment that was repeated two or more times with identical results. Accompanying graph shows the mean (± SEM) percentage of Smad2/3-positive nuclei observed in all photomicrographs. (B) Mv1Lu cells were incubated in the absence or presence of cycloheximide (10 μg/ml) for 30 min before stimulation with TGF-β1 (2.5 ng/ml) or purified bovine SPARC (30 μg/ml) for an additional 30 min at 37°C. Afterward, the cells were fixed and processed for indirect immunofluorescence with anti-Smad2/3 antibodies. Right, cycloheximide-mediated inhibition of Mv1Lu cell protein synthesis as measured by [35S]methionine in vivo labeling as described under MATERIALS AND METHODS.
Figure 7.
SPARC and SPARC-EC stimulate Smad2 phosphorylation in Mv1Lu and MB114 cells. (A–C) Mv1Lu or MB114 cells were stimulated for 0–120 min with 5 ng/ml TGF-β1 (TGF or T), or for 30 min with 30 μg/ml purified bovine SPARC (SP or S), recombinant SPARC-EC (EC), thrombospondin-1 (TSP), or 2 μg/ml BMP-7 (B) as indicated. The activation status of Smad2 and Smad1 was determined by immunoblot analysis by using phospho-specific Smad2 and Smad1 antibodies. Differences in protein loading were monitored by reprobing stripped membranes with either anti-Smad2 or -ERK1 antibodies. Data are from a representative experiment that was repeated twice with similar results.
Figure 8.
SPARC via its EC domain stimulates TGF-β–responsive reporter gene activity through a Smad3- and TGF-β receptor-dependent pathway. Mv1Lu or R1B cells were transiently transfected with p3TP-luciferase and pCMV-β-gal, together with or without dominant-negative Smad3/3A. The transfectants were stimulated with TGF-β1 (5 ng/ml), purified bovine SPARC (30 μg/ml), or recombinant SPARC-EC (30 μg/ml) for 18 h as indicated, and subsequently processed to measure luciferase and β-gal activities. Data are the mean (± SEM) luciferase activities of three independent experiments presented as the fold-stimulations of untreated cells.
Figure 9.
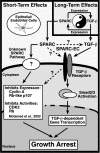
Schematic of SPARC-mediated regulation of and coupling to the TGF-β signaling system. Inset, long-term TGF-β treatment of Mv1Lu cells stimulates their synthesis of SPARC transcript and protein. Conversely, long-term SPARC treatment induces Mv1Lu cells to produce and secrete TGF-β1, presumably leading to enhanced autocrine TGF-β signaling. Dysregulation of this regulatory loop may exacerbate the development and progression of diseases characterized by alterations in tissue remodeling and repair, including cancer. Newly synthesized SPARC is also endowed with the potential to regulate short-term TGF-β signaling. Although it is unknown whether SPARC acts directly on TGF-β (i.e., activation of latent TGF-β complexes; dashed arrow) or its receptors (dashed arrow), these molecules are in fact necessary and required for SPARC to stimulate the TGF-β signaling system. Activation of TGF-β receptors by SPARC stimulates TGF-β–regulated Smads, which translocate into the nucleus to induce or repress TGF-β–responsive genes, ultimately leading to epithelial cell growth arrest. The ability of SPARC to couple to TGF-β signaling is mediated via its EC domain. Alternatively, cells resistant to TGF-β (i.e., loss of TGF-β receptors or Smad inactivation) remain competent to partial growth arrest by SPARC, a response that is not recapitulated by SPARC-EC. Activation of this unknown pathway likely inhibits the expression and activities of cell cycle components (e.g., cyclin A, Rb-like p107, CDK2, and Rb). Although the contribution of TGF-β signaling to regulation of this unknown SPARC pathway remains to be defined (dashed arrow), both systems cooperate to maximally inhibit epithelial cell proliferation by SPARC.
Similar articles
- SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells.
Francki A, McClure TD, Brekken RA, Motamed K, Murri C, Wang T, Sage EH. Francki A, et al. J Cell Biochem. 2004 Apr 1;91(5):915-25. doi: 10.1002/jcb.20008. J Cell Biochem. 2004. PMID: 15034927 - TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4.
Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P. Nakao A, et al. EMBO J. 1997 Sep 1;16(17):5353-62. doi: 10.1093/emboj/16.17.5353. EMBO J. 1997. PMID: 9311995 Free PMC article. - The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells.
Poncelet AC, de Caestecker MP, Schnaper HW. Poncelet AC, et al. Kidney Int. 1999 Oct;56(4):1354-65. doi: 10.1046/j.1523-1755.1999.00680.x. Kidney Int. 1999. PMID: 10504488 - Receptor-regulated Smads in TGF-beta signaling.
Liu F. Liu F. Front Biosci. 2003 Sep 1;8:s1280-303. doi: 10.2741/1149. Front Biosci. 2003. PMID: 12957874 Review. - New insights into TGF-beta-Smad signalling.
ten Dijke P, Hill CS. ten Dijke P, et al. Trends Biochem Sci. 2004 May;29(5):265-73. doi: 10.1016/j.tibs.2004.03.008. Trends Biochem Sci. 2004. PMID: 15130563 Review.
Cited by
- SPARC expression and prognostic value in non-small cell lung cancer.
Huang Y, Zhang J, Zhao YY, Jiang W, Xue C, Xu F, Zhao HY, Zhang Y, Zhao LP, Hu ZH, Yao ZW, Liu QY, Zhang L. Huang Y, et al. Chin J Cancer. 2012 Nov;31(11):541-8. doi: 10.5732/cjc.012.10212. Epub 2012 Oct 10. Chin J Cancer. 2012. PMID: 23114088 Free PMC article. - Efficacy and safety of weekly intravenous nanoparticle albumin-bound paclitaxel for non-small cell lung cancer patients who have failed at least two prior systemic treatments.
Duan J, Hao Y, Wan R, Yu S, Bai H, An T, Zhao J, Wang Z, Zhuo M, Wang J. Duan J, et al. Thorac Cancer. 2017 May;8(3):138-146. doi: 10.1111/1759-7714.12413. Epub 2017 Mar 17. Thorac Cancer. 2017. PMID: 28304139 Free PMC article. - The role of SPARC in extracellular matrix assembly.
Bradshaw AD. Bradshaw AD. J Cell Commun Signal. 2009 Dec;3(3-4):239-46. doi: 10.1007/s12079-009-0062-6. Epub 2009 Oct 2. J Cell Commun Signal. 2009. PMID: 19798598 Free PMC article. - SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-β1 activity.
Rivera LB, Brekken RA. Rivera LB, et al. J Cell Biol. 2011 Jun 27;193(7):1305-19. doi: 10.1083/jcb.201011143. J Cell Biol. 2011. PMID: 21708981 Free PMC article. - Role of taxanes in pancreatic cancer.
Belli C, Cereda S, Reni M. Belli C, et al. World J Gastroenterol. 2012 Sep 7;18(33):4457-65. doi: 10.3748/wjg.v18.i33.4457. World J Gastroenterol. 2012. PMID: 22969215 Free PMC article.
References
- Bassuk, J.A., et al. (2000). Induction of TGF-β by the matricellular protein SPARC in a rat model of glomerulonephritis. Kidney Int. 57, 117–128. - PubMed
- Blobe, G.C., Schiemann, W.P., and Lodish, H.F. (2000). Role of transforming growth factor β in human disease. N. Engl. J. Med. 342, 1350–1358. - PubMed
- Boyd, F.T., and Massague, J. (1989). Transforming growth factor-β inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J. Biol. Chem. 264, 2272–2278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous