The role of mVps18p in clustering, fusion, and intracellular localization of late endocytic organelles - PubMed (original) (raw)
The role of mVps18p in clustering, fusion, and intracellular localization of late endocytic organelles
Viviane Poupon et al. Mol Biol Cell. 2003 Oct.
Abstract
Delivery of endocytosed macromolecules to mammalian cell lysosomes occurs by direct fusion of late endosomes with lysosomes, resulting in the formation of hybrid organelles from which lysosomes are reformed. The molecular mechanisms of this fusion are analogous to those of homotypic vacuole fusion in Saccharomyces cerevisiae. We report herein the major roles of the mammalian homolog of yeast Vps18p (mVps18p), a member of the homotypic fusion and vacuole protein sorting complex. When overexpressed, mVps18p caused the clustering of late endosomes/lysosomes and the recruitment of other mammalian homologs of the homotypic fusion and vacuole protein sorting complex, plus Rab7-interacting lysosomal protein. The clusters were surrounded by components of the actin cytoskeleton, including actin, ezrin, and specific unconventional myosins. Overexpression of mVps18p also overcame the effect of wortmannin treatment, which inhibits membrane traffic out of late endocytic organelles and causes their swelling. Reduction of mVps18p by RNA interference caused lysosomes to disperse away from their juxtanuclear location. Thus, mVps18p plays a critical role in endosome/lysosome tethering, fusion, intracellular localization and in the reformation of lysosomes from hybrid organelles.
Figures
Figure 8.
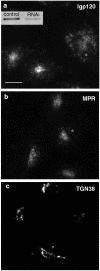
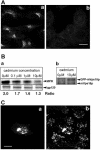
Reduction of intracellular mVps18p by RNA interference causes redistribution of lysosomes. NRK cells transiently transfected with mVps18p siRNA oligonucleotides (a–c) were processed for indirect immunofluorecence as in Figure 1, by using anti-lgp120 mAb GM10 (a), anti-MPR pAb1001 (b), or anti-TGN38 mAb 2F7.1 (c). Cells were observed using a conventional upright epifluorescence microscope. Stars, transfected cells. Bar, 10 μm. a, inset, expression of mVps18p was assessed on total cell lysates of untransfected (left) and transiently transfected (right) NRK cells by immunoblotting.
Figure 9.
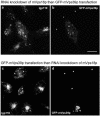
Reduction of intracellular mVps18p by RNA interference prevents mVps39p-induced clustering of lysosomes. NRK cells transiently transfected with mVps18p siRNA oligonucleotides then with a plasmid encoding GFP-mVps39p for 48h (a and b) or vice versa (c and d) were processed as in Figure 1, by using pAb 580 to lgp110 (a and c) and observed using a conventional upright epifluorescence microscope as in Figure 8. Single stars, cells transfected with mVps18 siRNAs. Double stars, cells transfected with both mVps18 siRNAs and the plasmid encoding GFP-mVps39p. Bar 10 μm. a and c, lgp110; b and d, GFP.
Figure 1.
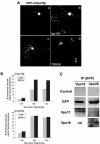
The overexpression of mVps18p causes clustering of lysosomes. (A) NRK cells grown on coverslips were transiently transfected and allowed to express GFP-mVps18p for 48h (a–d). Cells were permeabilized before fixation to wash out the cytosol and then labeled with anti-lgp120 mAb GM10 (a and b) or anti-TGN38 mAb 2F7.1 (c and d), and a Texas Red-labeled secondary antibody. Cells were then observed using a confocal microscope. a and c, green fluorescence emitted by GFP; b and d, red fluorescence emitted by Texas Red. Bar 10 μm. (B) NRK or HeLa cells grown on coverslips were transiently transfected and allowed to express GFP-mVps18 (left) or GFP-mVps39 (right) for 24, 48, and 72 h. Cells were labeled as in A with the anti-lgp120 mAb GM10. One hundred transfected cells were observed in each case, and the percentage of transfected cells with clearly visible lysosomal clustering was scored. Bar 10 μm. (C) NRK cells were transiently transfected and allowed to express GFP-mVps18p (left) or GFP-mVps39p (right) for 48 h. Cell lysates were immunoprecipitated (IP) with mAb 3B6 to GFP coupled to protein A-Sepharose beads. After elution, proteins were separated by SDS-PAGE and immunoblotted with mAb 11E5 to GFP or pAbs to mVps11p and mVps18p. The control panels are blots with the mAb to GFP after IP of lysates from pEGFPC1 transfected NRK cells. nd, not done. Vps11, 18, and 39 refer to mVps11p, mVps18p, and mVps39p, respectively.
Figure 2.
The lysosome clusters contain mammalian homologs of the HOPS complex components. NRK cells transiently transfected for 48h and expressing GFP-mVps18p (a–d) or GFP-mVps39p (e–j) were processed for confocal fluorescence microscopy as in Figure 1, by using pAb anti-mVps11p (a and b, g and h), anti-mVps18p (e and f), and anti-mVps33p (c and d, i and j) as primary antibodies. a, c, e, g, and i, GFP; b, mVps11p; d, mVps33p; f, mVps18p; h, mVps11p; and j, mVps33p. Bar, 10 μm.
Figure 3.
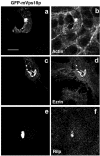
Actin, ezrin, and RILP are recruited into lysosome clusters. NRK cells transiently tranfected for 48 h and expressing GFP-mVps18p (a–f) were processed for confocal fluorescence microscopy, as in Figure 1, using Texas Red-labeled phalloidin (a and b), or pAb anti-ezrin (c and d), or pAb anti-Rilp (e and f). a, c, and e, GFP; b, phalloidin; d, ezrin; and f, RILP. Bar, 10 μm.
Figure 4.
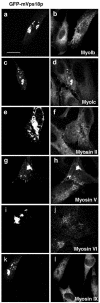
Mysosin proteins are recruited into lysosome clusters. NRK cells transiently tranfected for 48 h and expressing GFP-mVps18p (a–i) were processed for confocal fluorescence microscopy as in Figure 1, by using pAbs to MyoIb (a and b), MyoIc (c and d), Myosin II (e and f), Myosin V (g and h), Myosin VI (I and j) and Myosin IX (k and l) revealed by secondary Texas Red antibodies. a, c, e, g, i, and k, GFP; b, MyoIb; d, MyoIc; f, Myosin II; h, Myosin V; j, Myosin VI; and l, Myosin IX. Bar, 10 μm.
Figure 5.
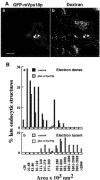
Endocytic uptake of dextran and BSA-gold. (A) NRK cells transiently transfected with GFP-mVps18p were incubated with Texas Red-dextran for 1 h and then fixed for confocal microscopy. a, GFP; b, Texas Red-dextran. Bar, 10 μm. (B) Stably transfected NRK cells, either uninduced or expressing GFP-mVps18p (as a result of induction with 10 μM cadmium chloride for 48 h), were incubated with BSA-gold 5 nm for 4 h followed by a 20-h chase (in the presence of 10 μM cadmium chloride) to label late endocytic organelles. Histograms show the area profile of electron dense and electron lucent organelles containing 5-nm gold in control (uninduced) cells and cells expressing GFP-mVps18p.
Figure 6.
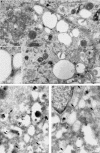
Electron microscopy of clustered late endocytic organelles. Stably transfected NRK cells expressing GFP-mVps18p were incubated with BSA-gold (5 nm) to load late endocytic organelles and then processed for conventional transmission electron microscopy (a and b) or immunoelectron microscopy (c and d). a, cluster of electron lucent organelles and dense core lysosomes surrounded by proteinaceous shell (open arrows). *(a, upper left quadrant), protein aggregates; small arrows, 5-nm gold; arrow heads, fine striations between adjacent organelles. Bar, 1 μm. b, enlargment (2.25×) of fine striations shown in a between arrow heads. c, immunogold labeling of lgp120 (15-nm gold, arrowheads) and MPR (10-nm gold, large arrows). Small arrows, 5-nm gold. Bar, 250 nm. d, immunogold labeling of lgp120 (15-nm gold, arrowheads) and GFP (10-nm gold, large arrows). Small arrows, 5-nm gold. Bar, 250 nm.
Figure 7.
Functional characterization of clusters of late endocytic organelles. (A) Overexpression of GFP-mVp18p disrupts MPR expression. Stably transfected NRK cells were not induced (a), or induced to express GFP-mVps18p for 48 h with 5 μM cadmium chloride (b), before processing for confocal fluorescence microscopy by using an anti-MPR pAb. a and b, MPR. (B) Immunoblotting of MPR. Stably transfected NRK cells were induced for 48 h with increasing amounts of cadmium chloride, ranging from 0.1 to 10 μM to stimulate expression of GFP-mVps18p. a, expression of MPR and lgp120; the ratios of densities of MPR compared with lgp120 bands are shown (the densities of lgp120 bands relative to density in noninduced cells are 89, 89, and 78%, and of MPR bands relative to density in noninduced cells are 76, 71, and 51% after induction, respectively, with 0.1, 1, and 10 μM cadmium chloride). b, expression of GFP-mVps18p and endogenous mVps18p were assessed in total cell lysates by immunoblotting. (C) Wortmannin treatment. NRK cells, transiently transfected for 48 h with GFP-mVps18p, were incubated with wortmannin for 45 min. The cells were fixed and processed for confocal fluorescence microscopy as described in MATERIALS AND METHODS. a, GFP, b, lgp120.
Figure 10.
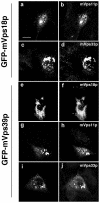
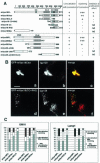
Delineation of functional domains in mVps18p and mVps39p. (A) Schematic representation of full-length mVps18p and mVps39p, and various deletion constructs. The columns on the right indicate whether constructs colocalize with lgp120, cause clustering of lysosomes, and prevent the effect of wortmannin on swelling of late endocytic organelles. (B) Expression of mVps18p deletion constructs. NRK cells transiently transfected for 48 h with GFP-tagged deletion constructs of mVps18p (a–f) were processed for confocal fluorescence microscopy, as in Figure 1, by using mAb anti-lgp120 (a–f) as a primary antibody. Herein, are shown the expression of two representative constructs, GFP-mVps18Cter (a–c), and GFP-mVps18(CC+RH2) (d–f). a and d, GFP; b and e, lgp120; c and f, merge of green and red channels. (C) Quantification of the effect of mVps18p and mVps39p constructs on localization and lysosome clustering. NRK (left) or HeLa cells (right) were transiently transfected for 48 h with all the GFP-tagged constructs of mVps18p and mVps39p and then processed for confocal fluorescence microscopy as in Figure 1, by using mAb anti-lgp120 for NRK cells and mAb anti-lamp1 for HeLa cells. For each construct, >100 cells were counted and sorted into three categories, according to colocalization with and clustering of lysosomes.
Similar articles
- Human Vam6p promotes lysosome clustering and fusion in vivo.
Caplan S, Hartnell LM, Aguilar RC, Naslavsky N, Bonifacino JS. Caplan S, et al. J Cell Biol. 2001 Jul 9;154(1):109-22. doi: 10.1083/jcb.200102142. J Cell Biol. 2001. PMID: 11448994 Free PMC article. - Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent.
Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio JP. Mullock BM, et al. J Cell Biol. 1998 Feb 9;140(3):591-601. doi: 10.1083/jcb.140.3.591. J Cell Biol. 1998. PMID: 9456319 Free PMC article. - Endosome-lysosome fusion.
Luzio JP, Gray SR, Bright NA. Luzio JP, et al. Biochem Soc Trans. 2010 Dec;38(6):1413-6. doi: 10.1042/BST0381413. Biochem Soc Trans. 2010. PMID: 21118098 - Relationship between endosomes and lysosomes.
Luzio JP, Mullock BM, Pryor PR, Lindsay MR, James DE, Piper RC. Luzio JP, et al. Biochem Soc Trans. 2001 Aug;29(Pt 4):476-80. doi: 10.1042/bst0290476. Biochem Soc Trans. 2001. PMID: 11498012 Review. - Membrane traffic to and from lysosomes.
Luzio JP, Pryor PR, Gray SR, Gratian MJ, Piper RC, Bright NA. Luzio JP, et al. Biochem Soc Symp. 2005;(72):77-86. doi: 10.1042/bss0720077. Biochem Soc Symp. 2005. PMID: 15649132 Review.
Cited by
- Recruitment of VPS33A to HOPS by VPS16 Is Required for Lysosome Fusion with Endosomes and Autophagosomes.
Wartosch L, Günesdogan U, Graham SC, Luzio JP. Wartosch L, et al. Traffic. 2015 Jul;16(7):727-42. doi: 10.1111/tra.12283. Epub 2015 Apr 30. Traffic. 2015. PMID: 25783203 Free PMC article. - Structural basis of Vps33A recruitment to the human HOPS complex by Vps16.
Graham SC, Wartosch L, Gray SR, Scourfield EJ, Deane JE, Luzio JP, Owen DJ. Graham SC, et al. Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13345-50. doi: 10.1073/pnas.1307074110. Epub 2013 Jul 30. Proc Natl Acad Sci U S A. 2013. PMID: 23901104 Free PMC article. - The small GTPase Arl8b regulates assembly of the mammalian HOPS complex on lysosomes.
Khatter D, Raina VB, Dwivedi D, Sindhwani A, Bahl S, Sharma M. Khatter D, et al. J Cell Sci. 2015 May 1;128(9):1746-61. doi: 10.1242/jcs.162651. Epub 2015 Apr 23. J Cell Sci. 2015. PMID: 25908847 Free PMC article. - Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking.
Shohdy N, Efe JA, Emr SD, Shuman HA. Shohdy N, et al. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4866-71. doi: 10.1073/pnas.0501315102. Epub 2005 Mar 21. Proc Natl Acad Sci U S A. 2005. PMID: 15781869 Free PMC article. - Vps11 and Vps18 of Vps-C membrane traffic complexes are E3 ubiquitin ligases and fine-tune signalling.
Segala G, Bennesch MA, Ghahhari NM, Pandey DP, Echeverria PC, Karch F, Maeda RK, Picard D. Segala G, et al. Nat Commun. 2019 Apr 23;10(1):1833. doi: 10.1038/s41467-019-09800-y. Nat Commun. 2019. PMID: 31015428 Free PMC article.
References
- Barois, N., Forquet, F., and Davoust, J. (1998). Actin microfilaments control the MHC class II antigen presentation pathway in B cells. J. Cell Sci. 111, 1791–1800. - PubMed
- Borden, K.L., and Freemont, P.S. (1996). The RING finger domain: a recent example of a sequence-structure family. Curr. Opin. Struct. Biol. 6, 395–401. - PubMed
- Bretscher, A. (1999). Regulation of cortical structure by the ezrin-radixinmoesin protein family. Curr. Opin. Cell Biol. 11, 109–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases