Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis - PubMed (original) (raw)
Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis
Laurent Waselle et al. Mol Biol Cell. 2003 Oct.
Abstract
Rab27a is a GTPase associated with insulin-containing secretory granules of pancreatic beta-cells. Selective reduction of Rab27a expression by RNA interference did not alter granule distribution and basal secretion but impaired exocytosis triggered by insulin secretagogues. Screening for potential effectors of the GTPase revealed that the Rab27a-binding protein Slac2c/MyRIP is associated with secretory granules of beta-cells. Attenuation of Slac2c/MyRIP expression by RNA interference did not modify basal secretion but severely impaired hormone release in response to secretagogues. Although beta-cells express Myosin-Va, a potential partner of Slac2c/MyRIP, no functional link between the two proteins could be demonstrated. In fact, overexpression of the Myosin-Va binding domain of Slac2c/MyRIP did not affect granule localization and hormone exocytosis. In contrast, overexpression of the actin-binding domain of Slac2c/MyRIP led to a potent inhibition of exocytosis without detectable alteration in granule distribution. This effect was prevented by point mutations that abolish actin binding. Taken together our data suggest that Rab27a and Slac2c/MyRIP are part of a complex mediating the interaction of secretory granules with cortical actin cytoskeleton and participate to the regulation of the final steps of insulin exocytosis.
Figures
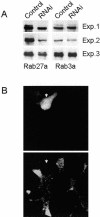
Figure 1.
Rab27a expression can be selectively decreased by RNA interference. (A) INS-1E cells were transiently transfected with GFP-tagged Rab27a or with GFP-tagged Rab3a. Silencing of the genes was determined by cotransfecting the cells either with an empty pSUPER vector (control) or with a pSUPER vector containing a sequence that directs the synthesis rat Rab27a-specific siRNAs (RNAi). The cells were homogenized and analyzed by Western blotting using an anti-GFP antibody. The figure shows the results of three independent experiments. (B) INS-1E cells were transiently cotransfected with GFP and with the Rab27a silencer. After three days the cells were fixed and analyzed by immunofluorescence using an antibody against Rab27a. Top: GFP fluorescence. Bottom: expression of endogenous Rab27a. The arrow indicates the position of a GFP-positive cell expressing the silencer.
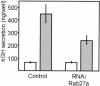
Figure 2.
Effect of Rab27a knock down on exocytosis. INS-1E cells were transiently transfected with a plasmid encoding hGH and with either the empty vector (control) or a with the vector containing a sequence that directs the synthesis of Rab27a-specific siRNAs (RNAi). Three days later the cells were incubated under basal (□) or stimulatory (▪) conditions. The amount of hGH released during the incubation period was determined by ELISA.
Figure 3.
A decrease in Rab27a function does not affect the distribution of insulin-containing granules. INS-1E cells were transiently transfected with the Rab27a silencer together with GFP (a and b) or with GFP-tagged Rab27a T23N (c and d). Three days later they were fixed and analyzed by confocal microscopy. Transfected cells were identified by the GFP fluorescence (a and c). The distribution of secretory granules was visualized using an antibody against insulin (b and d).
Figure 4.
Screening for members of the Slp family expressed in insulin-secreting cell lines. Total homogenates of INS-1E, HIT-T15, and βTC3 were analyzed by Western blotting using antibodies against Slp1, Slp2a, Slp3a, Slp5, Slac2a, and Slac2c. When no detectable signal was obtained in insulin-secreting cells, homogenates of COS cells transfected with the appropriate T7-tagged Slps were included as a positive control.
Figure 5.
Slac2c/MyRIP is expressed in pancreatic islets. Mouse pancreatic sections were incubated with a polyclonal antibody directed against Slac2c/MyRIP. The presence of immunocomplexes was revealed using a Cy3-labeled anti-rabbit antibody. The image was obtained by confocal microscopy.
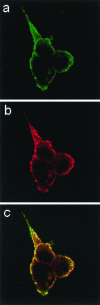
Figure 6.
Subcellular localization of Slac2c/MyRIP. INS-1E cells grown on glass coverslips were fixed with paraformaldehyde and labeled with a guinea pig antibody against insulin and a rabbit antibody against Slac2c/MyRIP. Images were obtained by confocal microscopy after incubation of the coverslips with an Oregon-green–coupled anti–guinea pig antibody and a Cy3-coupled anti-rabbit antibody. (a) The localization of insulin; (b) the position of Slac2c/MyRIP; (c) the superposition of the green and red channels.
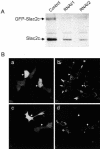
Figure 7.
Slac2c/MyRIP expression can be selectively decreased by RNA interference. (A) INS-1E cells were transiently transfected with GFP-tagged Slac2c. Silencing of the genes was determined by cotransfecting the cells either with an empty vector (control) or with vectors containing sequences that direct the synthesis Slac2c/MyRIP-specific siRNAs (RNAi/1 and RNAi/2). Three days later the cells were homogenized and equal amounts of proteins were analyzed by Western blotting using an anti-Slac2c antibody. The positions of endogenous Slac2c and of its transfected GFP-tagged counterpart are indicated by the arrows. (B) INS-1E cells were transiently cotransfected with GFP and with an empty vector (a and b) or with a vector containing the RNAi/2 silencer sequence (c and d). After 3 d the cells were fixed and analyzed by immunofluorescence using the antibody against Slac2c/MyRIP. The arrows indicate the position of transiently transfected cells expressing the silencer.
Figure 8.
Effect of Slac2c/MyRIP knock down on INS-1E exocytosis. INS-1E cells were transiently transfected with plasmids that encode hGH and contain sequences under the control of an H1-promoter that direct the synthesis Slac2c/MyRIP-specific siRNAs. A vector encoding hGH but devoid of RNAi-inducing sequences was used as a control. Three days later the cells were incubated under basal (□) or stimulatory (▪) conditions. The amount of hGH released during the incubation period was determined by ELISA.
Figure 9.
Expression of unconventional myosins in pancreatic islets. Mouse pancreatic sections were incubated with antibodies directed against Myosin Va (a) or Myosin VIIa (c) and with an antibody against insulin (b and d). Images were obtained by confocal microscopy.
Figure 10.
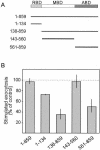
Effect of Slac2c/MyRIP constructs on INS-1E exocytosis. (A) Schematic representation of Slac2c/MyRIP and of the deletion mutants used in this study. The numbers on the left indicate the amino acids included in each construct. RBD, Rab-binding domain; MBD, Myosin-binding domain; ABD, actin-binding domain. (B) The different Slac2c/MyRIP constructs were transiently cotransfected in INS-1E cells together with a plasmid encoding hGH. Three days later the cells were incubated under basal or stimulatory conditions. The amount of hGH released under each condition was determined by ELISA. None of the constructs affected basal hGH secretion. The figure shows the secretory response of the cells under stimulatory conditions. The results correspond to the mean ± SEM of three independent experiments performed in triplicates. The amount of hormone released under stimulatory conditions from cells cotransfected with the hGH plasmid and an empty vector was set to 100%.
Figure 11.
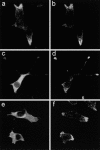
Subcellular distribution of Slac2c/MyRIP constructs in INS-1E cells. INS-1E cells were transiently transfected with myc-tagged constructs encoding the Rab-binding domain (a and b), the Myosin-binding domain (c and d) or the actin-binding domain (e and f). Two days later the cells were fixed, double stained with antibodies directed against the myc tag (a, c, and e) and against insulin (b, d, and f), and analyzed by confocal microscopy.
Figure 12.
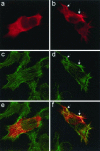
The C-terminal domain of Slac2c/MyRIP colocalizes with cortical actin cytoskeleton. INS-1E cells were transiently transfected with the Myosin-binding domain (a, c, and e) or the actin-binding domain (b, d, and f) of Slac2c/MyRIP. Two days later the cells were fixed and stained with an antimyc antibody (a and b) and with fluorescently labeled phalloidin (c and d). Images were taken using confocal microscopy. Panels e and f correspond to the overlay of the antimyc and phalloidin signal. The arrows indicate regions where the C-terminus of Slac2c/MyRIP and actin filaments colocalize.
Figure 13.
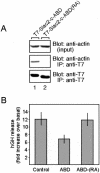
The actin binding activity of the C-terminus of Slac2c is required for the control of exocytosis. (A) The Slac2c-ABD(RA) mutant lacks actin-binding activity. T7-Slac2c-ABD and the Slac2c-ABD(RA) mutant were expressed in COS-7 cells and immunoprecipitated with anti-T7 tag antibody-conjugated agarose. Coimmunoprecipitated actin was first detected by antiactin antibody (middle panel) and the immunoprecipitated T7-Slac2c-ABD proteins were then visualized with anti-T7 tag antibody (bottom panel). The top panel shows total expressed proteins (1/80 volume; input) used for immunoprecipitation. (B) INS1-E cells were transiently cotransfected with a plasmid encoding hGH and with T7-Slac2c-ABD, Slac2c-ABD(RA) or with an empty vector (control). Three days later the cells were incubated under basal or under stimulatory conditions. The results show the ratio between the amount of hGH released under basal and stimulatory conditions.
Similar articles
- Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites.
Desnos C, Schonn JS, Huet S, Tran VS, El-Amraoui A, Raposo G, Fanget I, Chapuis C, Ménasché G, de Saint Basile G, Petit C, Cribier S, Henry JP, Darchen F. Desnos C, et al. J Cell Biol. 2003 Nov 10;163(3):559-70. doi: 10.1083/jcb.200302157. J Cell Biol. 2003. PMID: 14610058 Free PMC article. - The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis.
Cheviet S, Coppola T, Haynes LP, Burgoyne RD, Regazzi R. Cheviet S, et al. Mol Endocrinol. 2004 Jan;18(1):117-26. doi: 10.1210/me.2003-0300. Epub 2003 Oct 30. Mol Endocrinol. 2004. PMID: 14593078 - Exophilin8 transiently clusters insulin granules at the actin-rich cell cortex prior to exocytosis.
Mizuno K, Ramalho JS, Izumi T. Mizuno K, et al. Mol Biol Cell. 2011 May 15;22(10):1716-26. doi: 10.1091/mbc.E10-05-0404. Epub 2011 Mar 25. Mol Biol Cell. 2011. PMID: 21441305 Free PMC article. - Rab27a, actin and beta-cell endocytosis.
Kimura T, Niki I. Kimura T, et al. Endocr J. 2011;58(1):1-6. doi: 10.1507/endocrj.k10e-391. Epub 2010 Dec 23. Endocr J. 2011. PMID: 21187662 Review. - The role of myosin Va in secretory granule trafficking and exocytosis.
Eichler TW, Kögel T, Bukoreshtliev NV, Gerdes HH. Eichler TW, et al. Biochem Soc Trans. 2006 Nov;34(Pt 5):671-4. doi: 10.1042/BST0340671. Biochem Soc Trans. 2006. PMID: 17052171 Review.
Cited by
- Interplay between Rab27a effectors in pancreatic β-cells.
Yamaoka M, Ishizaki T, Kimura T. Yamaoka M, et al. World J Diabetes. 2015 Apr 15;6(3):508-16. doi: 10.4239/wjd.v6.i3.508. World J Diabetes. 2015. PMID: 25897360 Free PMC article. Review. - Overexpression of synaptic vesicle protein Rab GTPase 3C promotes vesicular exocytosis and drug resistance in colorectal cancer cells.
Chang YC, Li CH, Chan MH, Fang CY, Zhang ZX, Chen CL, Hsiao M. Chang YC, et al. Mol Oncol. 2023 Mar;17(3):422-444. doi: 10.1002/1878-0261.13378. Epub 2023 Feb 14. Mol Oncol. 2023. PMID: 36652260 Free PMC article. - Role of Rab27 in synaptic transmission at the squid giant synapse.
Yu E, Kanno E, Choi S, Sugimori M, Moreira JE, Llinás RR, Fukuda M. Yu E, et al. Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):16003-8. doi: 10.1073/pnas.0804825105. Epub 2008 Oct 7. Proc Natl Acad Sci U S A. 2008. PMID: 18840683 Free PMC article. - Filamentous actin regulates insulin exocytosis through direct interaction with Syntaxin 4.
Jewell JL, Luo W, Oh E, Wang Z, Thurmond DC. Jewell JL, et al. J Biol Chem. 2008 Apr 18;283(16):10716-26. doi: 10.1074/jbc.M709876200. Epub 2008 Feb 19. J Biol Chem. 2008. PMID: 18285343 Free PMC article. - ICER induced by hyperglycemia represses the expression of genes essential for insulin exocytosis.
Abderrahmani A, Cheviet S, Ferdaoussi M, Coppola T, Waeber G, Regazzi R. Abderrahmani A, et al. EMBO J. 2006 Mar 8;25(5):977-86. doi: 10.1038/sj.emboj.7601008. Epub 2006 Feb 23. EMBO J. 2006. PMID: 16498408 Free PMC article.
References
- Asfari, M., Janjic, D., Meda, P., Li, G., Halban, P.A., and Wollheim, C.B. (1992). Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130, 167–178. - PubMed
- Brummelkamp, T.R., Bernards, R., and Agami, R. (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553. - PubMed
- Chen, D., Guo, J., Miki, T., Tachibana, M., and Gahl, W.A. (1997). Molecular cloning and characterization of Rab27a and Rab27b, novel human Rab proteins shared by melanocytes and platelets. Biochem. Mol. Med. 60, 27–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases