The retrieval function of the KDEL receptor requires PKA phosphorylation of its C-terminus - PubMed (original) (raw)
The retrieval function of the KDEL receptor requires PKA phosphorylation of its C-terminus
Margarita Cabrera et al. Mol Biol Cell. 2003 Oct.
Erratum in
- Mol Biol Cell. 2004 Jan;15(1):preceding Table of Contents
Abstract
The KDEL receptor is a Golgi/intermediate compartment-located integral membrane protein that carries out the retrieval of escaped ER proteins bearing a C-terminal KDEL sequence. This occurs throughout retrograde traffic mediated by COPI-coated transport carriers. The role of the C-terminal cytoplasmic domain of the KDEL receptor in this process has been investigated. Deletion of this domain did not affect receptor subcellular localization although cells expressing this truncated form of the receptor failed to retain KDEL ligands intracellularly. Permeabilized cells incubated with ATP and GTP exhibited tubular processes-mediated redistribution from the Golgi area to the ER of the wild-type receptor, whereas the truncated form lacking the C-terminal domain remained concentrated in the Golgi. As revealed with a peptide-binding assay, this domain did not interact with both coatomer and ARF-GAP unless serine 209 was mutated to aspartic acid. In contrast, alanine replacement of serine 209 inhibited coatomer/ARF-GAP recruitment, receptor redistribution into the ER, and intracellular retention of KDEL ligands. Serine 209 was phosphorylated by both cytosolic and recombinant protein kinase A (PKA) catalytic subunit. Inhibition of endogenous PKA activity with H89 blocked Golgi-ER transport of the native receptor but did not affect redistribution to the ER of a mutated form bearing aspartic acid at position 209. We conclude that PKA phosphorylation of serine 209 is required for the retrograde transport of the KDEL receptor from the Golgi complex to the ER from which the retrieval of proteins bearing the KDEL signal depends.
Figures
Figure 1.
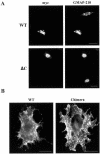
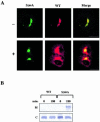
Immunofluorescence localization of KDEL and CXCR4 receptors. (A) COS cells were transfected with plasmid HE24M coding a version of the KDEL receptor containing a myc epitope inserted between the last transmembrane domain and the C-terminal cytoplasmic domain. Cells expressing either the intact, wild-type receptor (WT) or a truncated form lacking the last 12 amino acids (ΔC) were double-stained with anti-myc and anti-GMAP-210 antibodies. (B) COS cells were transfected with a plasmid coding either wild-type CXCR4 (WT) or a chimera resulting from the replacement of the last 41 amino acids of this protein with the C-terminal domain of the KDEL receptor. They were single-stained with anti-CXCR4 antibody. Bars, 20 μm.
Figure 2.
Secretion of lysozyme-KDEL. (A) COS cells were transfected with a plasmid coding hen lysozyme containing the KDEL signal at the C terminus. (B) Alternatively, they were transfected with a plasmid coding both lysozyme-KDEL and a particular myc-tagged version of the KDEL receptor, either the intact, wild-type receptor (WT) or a truncated form lacking the last 12 amino acids (ΔC). Cells were pulse-labeled for 10 min with [35S]methionine and -cysteine and chased for the indicated time periods. At each time point, lysozyme-KDEL was immunoprecipitated from both medium (M) and cell pellet (C) and resolved by SDS-PAGE.
Figure 3.
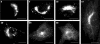
Golgi-ER redistribution of the native KDEL receptor. Vero cells were permeabilized with SLO, rinsed with buffer, and incubated at 37°C for the indicated time periods (min) with 1 mM of both ATP and GTP. Cells were fixed and processed for indirect immunofluorescence with an antibody against the C-terminal domain of the KDEL receptor. Bars, 20 μm
Figure 4.
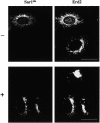
Golgi-ER redistribution of different fluorescent KDEL receptor constructs. COS cells were transfected with plasmid coding a CFP-tagged version of either the wild-type KDEL receptor (WT) or a truncated form lacking the last 12 amino acids (ΔC). They were permeabilized with SLO and incubated at 37°C for 35 min either in the presence (+) or in the absence (–) of both ATP and GTP. Cells were fixed and processed for indirect immunofluorescence with an antibody against the C-terminal domain of the KDEL receptor. Bars, 16 μm
Figure 5.
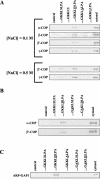
Interaction of native coatomer proteins and ARF-GAP with peptides. Synthetic peptides were covalently coupled to thiopropyl Sepharose beads. They were incubated with crude bovine brain cytosol for 5 min at room temperature, rinsed with buffer, and processed by SDS-PAGE and immunoblotting. The same Western blot membrane was reused to detect several coatomer proteins. Incubation with cytosol and rinses with buffer were carried out at the indicated NaCl concentrations (A) or, alternatively, at 0.1 M NaCl (B and C). Peptide... GKKLSLPA corresponds to the entire C-terminal domain of the native KDEL receptor. Changes on this sequence are indicated as underlined residues and the caret indicates deletion). As a negative control, beads with no peptide coupled were similarly processed (control). Also, 20 μg of crude cytosol was processed and loaded on gels as a positive control (cytosol).
Figure 6.
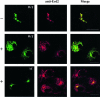
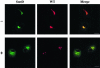
Effects of S209A replacement on receptor redistribution and secretion of lysozyme-KDEL. (A) COS cells were cotransfected with two plasmids, one coding a YFP-tagged version of the wild-type KDEL receptor and the other coding a CFP-tagged version of a mutant form in which S209 was changed to A (S209A). Cells were permeabilized and incubated at 37°C for 25 min either in the presence (+) or in the absence (–) of both ATP and GTP before fixation. Bars, 16 μm. (B) COS cells were transfected with a plasmid coding both lysozyme-KDEL and the myc-tagged version of the KDEL receptor. Cells expressing either the wild-type receptor (WT) or the S209A mutant form were pulse-labeled for 10 min and chased for 3 h. Lysozyme-KDEL was immunoprecipitated from both medium (M) and cell pellet (C) and resolved by SDS-PAGE.
Figure 7.
PKA phosphorylation of the KDEL receptor. Microsomal membranes containing myc-tagged versions of the KDEL receptor were prepared from baculovirus-infected insect cells, washed with high-salt buffer, and subjected to PKA-mediated phosphorylation. (A) Membranes containing either the wild-type KDEL receptor (WT) or the S209A mutant form were incubated at 30°C for 10 min with [γ-32P]ATP either in the presence or in the absence (–) of 1 U Cα. (B) Membranes containing the wild-type KDEL receptor were similarly incubated with both [γ-32P]ATP and 100 μg rat liver cytosol. The latter was preincubated for 10 min with both phosphatase inhibitors and the indicated concentrations of recombinant RIIα. Membranes were rinsed with buffer and lysed with detergents before immunoprecipitation of the KDEL receptor with anti-myc antibody. Immunoprecipitates were resolved by SDS-PAGE and either directly visualized in PhosphorImager (B) or previously transferred to Western blot membranes (A). Radioactive (32P) bands in A were identified by immunoblotting with anti-myc antibody. In this case, protein G-Sepharose beads with anti-myc antibody covalently cross-linked were used during immunoprecipitation to avoid interference of immunoglobulin light chains during immunoblotting.
Figure 8.
Effect of H89 treatment on the Golgi-ER redistribution of the native KDEL receptor. Vero cells were preincubated (+) or not (–) for 10–15 min with 5 μM H89 in FCS-free medium supplemented with 25 mM HEPES. They were microinjected in the same medium with recombinant YFP-tagged Sar1dn and incubation continued at 37°C for 1 h. Cells were fixed and processed for indirect immunofluorescence with an antibody against the KDEL receptor. Bars, 16 μm
Figure 9.
Effect of H89 treatment on the Golgi-ER redistribution of the S209D mutant form of the KDEL receptor. Vero cells were cotransfected with two plasmids, one coding a YFP-tagged version of the wild-type KDEL receptor and the other coding a CFP-tagged version of a mutant form in which S209 was changed to D (S209D). Twenty-four hours after transfection they were incubated or not (–) at 37°C for 1 h with 20 μM H89 in FCS-free medium before fixation. Bars, 16 μm
Similar articles
- Pathogenic Effects of Impaired Retrieval between the Endoplasmic Reticulum and Golgi Complex.
Kokubun H, Jin H, Aoe T. Kokubun H, et al. Int J Mol Sci. 2019 Nov 9;20(22):5614. doi: 10.3390/ijms20225614. Int J Mol Sci. 2019. PMID: 31717602 Free PMC article. Review. - Modulation of intracellular transport by transported proteins: insight from regulation of COPI-mediated transport.
Aoe T, Lee AJ, van Donselaar E, Peters PJ, Hsu VW. Aoe T, et al. Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1624-9. doi: 10.1073/pnas.95.4.1624. Proc Natl Acad Sci U S A. 1998. PMID: 9465066 Free PMC article. - Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum.
Girod A, Storrie B, Simpson JC, Johannes L, Goud B, Roberts LM, Lord JM, Nilsson T, Pepperkok R. Girod A, et al. Nat Cell Biol. 1999 Nov;1(7):423-30. doi: 10.1038/15658. Nat Cell Biol. 1999. PMID: 10559986 - KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET.
Majoul I, Straub M, Hell SW, Duden R, Söling HD. Majoul I, et al. Dev Cell. 2001 Jul;1(1):139-53. doi: 10.1016/s1534-5807(01)00004-1. Dev Cell. 2001. PMID: 11703931 - COPs regulating membrane traffic.
Kreis TE, Lowe M, Pepperkok R. Kreis TE, et al. Annu Rev Cell Dev Biol. 1995;11:677-706. doi: 10.1146/annurev.cb.11.110195.003333. Annu Rev Cell Dev Biol. 1995. PMID: 8689572 Review.
Cited by
- The physiology of membrane transport and endomembrane-based signalling.
Sallese M, Pulvirenti T, Luini A. Sallese M, et al. EMBO J. 2006 Jun 21;25(12):2663-73. doi: 10.1038/sj.emboj.7601172. Epub 2006 Jun 8. EMBO J. 2006. PMID: 16763561 Free PMC article. Review. - Pathogenic Effects of Impaired Retrieval between the Endoplasmic Reticulum and Golgi Complex.
Kokubun H, Jin H, Aoe T. Kokubun H, et al. Int J Mol Sci. 2019 Nov 9;20(22):5614. doi: 10.3390/ijms20225614. Int J Mol Sci. 2019. PMID: 31717602 Free PMC article. Review. - Regulation of Golgi signaling and trafficking by the KDEL receptor.
Cancino J, Jung JE, Luini A. Cancino J, et al. Histochem Cell Biol. 2013 Oct;140(4):395-405. doi: 10.1007/s00418-013-1130-9. Epub 2013 Jul 20. Histochem Cell Biol. 2013. PMID: 23873287 Review. - Directing Traffic: Regulation of COPI Transport by Post-translational Modifications.
Luo PM, Boyce M. Luo PM, et al. Front Cell Dev Biol. 2019 Sep 11;7:190. doi: 10.3389/fcell.2019.00190. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31572722 Free PMC article. Review. - Crosstalk between KDEL receptor and EGF receptor mediates cell proliferation and migration via STAT3 signaling.
Jia J, Zhu L, Yue X, Tang S, Jing S, Tan C, Du Y, Gao J, Lee I, Qian Y. Jia J, et al. Cell Commun Signal. 2024 Feb 20;22(1):140. doi: 10.1186/s12964-024-01517-w. Cell Commun Signal. 2024. PMID: 38378560 Free PMC article.
References
- Amara, A., Gall, S.L., Schwartz, O., Salamero, J., Montes, M., Loetscher, P., Baggiolini, M., Virelizier, J.L., and Arenzana-Seisdedos, F. (1997). HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186, 139–146. - PMC - PubMed
- Aridor, M., and Balch, W.E. (2000). Kinase signaling initiates coat complex II (COPII) recruitment and export from mammalian endoplasmic reticulum. J. Biol. Chem. 275, 35673–35676. - PubMed
- Barlowe, C. (2000). Traffic COPs of the early secretory pathway. Traffic 1, 371–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous