Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury - PubMed (original) (raw)
Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury
Richard M Lovering et al. Am J Physiol Cell Physiol. 2004 Feb.
Abstract
The purpose of this study was to evaluate the integrity of the muscle membrane and its associated cytoskeleton after a contraction-induced injury. A single eccentric contraction was performed in vivo on the tibialis anterior (TA) of male Sprague-Dawley rats at 900 degrees /s throughout a 90 degrees -arc of motion. Maximal tetanic tension (Po) of the TAs was assessed immediately and at 3, 7, and 21 days after the injury. To evaluate sarcolemmal integrity, we used an Evans blue dye (EBD) assay, and to assess structural changes, we used immunofluorescent labeling with antibodies against contractile (myosin, actin), cytoskeletal (alpha-actinin, desmin, dystrophin, beta-spectrin), integral membrane (alpha- and beta-dystroglycan, sarcoglycan), and extracellular (laminin, fibronectin) proteins. Immediately after injury, P0 was significantly reduced to 4.23 +/- 0.22 N, compared with 8.24 +/- 1.34 N in noninjured controls, and EBD was detected intracellularly in 54 +/- 22% of fibers from the injured TA, compared with 0% in noninjured controls. We found a significant association between EBD-positive fibers and the loss of complete dystrophin labeling. The loss of dystrophin was notable because organization of other components of the subsarcolemmal cytoskeleton was affected minimally (beta-spectrin) or not at all (alpha- and beta-dystroglycan). Labeling with specific antibodies indicated that dystrophin's COOH terminus was selectively more affected than its rod domain. Twenty-one days after injury, contractile properties were normal, fibers did not contain EBD, and dystrophin organization and protein level returned to normal. These data indicate the selective vulnerability of dystrophin after a single eccentric contraction-induced injury and suggest a critical role of dystrophin in force transduction.
Figures
Fig. 1
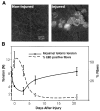
Membrane damage with loss of function. A: integrity of the sarcolemma was assessed by permeation of Evans blue dye (EBD). Noninjured TAs did not show dye uptake into the fibers (left), whereas injured TAs did (right), indicating sarcolemma damage. B: the percentage of EBD-positive fibers (dashed line) is plotted against contractile function (solid line), as measured by maximal isometric tetanic tension (Po). EBD was found in a high percentage of fibers immediately after the injury, when Po dropped significantly from noninjured values. Minimal EBD was seen 21 days postinjury, when contractile function was fully restored.
Fig. 2
Labeling of membrane-associated cytoskeleton after injury. Tissue sections from injured tibialis anterior muscles (TAs) were labeled for laminin (A–C), α-dystroglycan (D–F), and β-dystroglycan (G–I). The left column shows images of the respective molecules of interest. EBD-positive fibers from the same section are shown in the middle column, and the right column shows the overlay of the EDB (red) with fluorescent labeling (green). EBD was administered by intraperitoneal injection 24 h before injury. The data indicate that the organization of membrane-associated molecules is not affected. Scale bar = 50 μm.
Fig. 3
Labeling of the subsarcolemmal cytoskeleton after injury. Tissue sections from injured TAs were labeled for dystrophin (AC), β-spectrin (D–F), and desmin (G–I). The left column shows images of the respective molecules of interest. EBD-positive fibers from the same section are shown in the middle column, and the right column shows the overlay of the EDB (red) with fluorescent labeling (green). EBD was administered by intraperitoneal injection 24 h before injury. Fibers that were permeable to EBD also lacked dystrophin (arrows, A–C). β-Spectrin and desmin were much less affected in injured fibers (arrows, D–F and G–I, respectively). Scale bar = 50 μm.
Fig. 4
Double labeling of dystrophin and desmin. Fluorescent confocal images (×25) from double labeling of dystrophin and desmin of uninjured (A–C) and injured (D–F) tissue sections. Sections were labeled with monoclonal antibodies against dystrophin (B and E and light blue in overlay C or yellow/green in overlay F) and polyclonal antibodies against desmin (A and D and blue in C and F). In noninjured muscle, desmin labeling is throughout the fibers and dystrophin labeling is circumferential as expected. In injured fibers, loss of desmin labeling is more diffuse (asterisk in D), and there is incomplete labeling of dystrophin (arrow in E). Scale bar = 25 μm.
Fig. 5
Loss of dystrophin labeling is confined to the COOH terminus. Serial sections from an injured TA are labeled with monoclonal antibodies to either the rod domain (dys1, A) or the COOH terminus (dys2, B), showing a selective loss of labeling to the COOH terminus. Scale bar = 50 μm.
Fig. 6
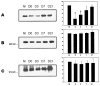
Western blot analysis of dystrophin and desmin from injured TA muscles. Western blot analysis was performed on injured TA at various time points after injury. NI are noninjured TAs, and D0 represents TAs harvested within 15 min postinjury. Data are also included for 3, 7, and 21 days after injury (D3, D7, D21, respectively). Each lane contains 20 μg of protein. A significant drop in the chemiluminescent signal is seen for dystrophin after injury (A). Although modulated, desmin does not show the same loss (B). The nonmembrane protein α-actinin was used as a control (C). Each bar represents data from 3 independent experiments, and the histograms represent means ± SD.
Fig. 7
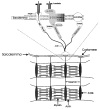
Schematic of selected membrane-associated molecules.
Similar articles
- [Changes of dystrophin and desmin in rat gastrocnemius under micro-damage induced by hypoxia].
Xu YM, Li JP, Wang RY. Xu YM, et al. Sheng Li Xue Bao. 2010 Aug 25;62(4):339-48. Sheng Li Xue Bao. 2010. PMID: 20717635 Chinese. - Loss of dystrophin and some dystrophin-associated proteins with concomitant signs of apoptosis in rat leg muscle overworked in extension.
Biral D, Jakubiec-Puka A, Ciechomska I, Sandri M, Rossini K, Carraro U, Betto R. Biral D, et al. Acta Neuropathol. 2000 Dec;100(6):618-26. doi: 10.1007/s004010000231. Acta Neuropathol. 2000. PMID: 11078213 - Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components.
Fridén J, Lieber RL. Fridén J, et al. Acta Physiol Scand. 2001 Mar;171(3):321-6. doi: 10.1046/j.1365-201x.2001.00834.x. Acta Physiol Scand. 2001. PMID: 11412144 Review. - Ultrastructural localization of alpha-, beta- and gamma-sarcoglycan and their mutual relation, and their relation to dystrophin, beta-dystroglycan and beta-spectrin in normal skeletal myofiber.
Wakayama Y, Inoue M, Kojima H, Murahashi M, Shibuya S, Jimi T, Hara H, Oniki H. Wakayama Y, et al. Acta Neuropathol. 1999 Mar;97(3):288-96. doi: 10.1007/s004010050987. Acta Neuropathol. 1999. PMID: 10090677
Cited by
- Early metabolic changes measured by 1H MRS in healthy and dystrophic muscle after injury.
Xu S, Pratt SJP, Spangenburg EE, Lovering RM. Xu S, et al. J Appl Physiol (1985). 2012 Sep 1;113(5):808-16. doi: 10.1152/japplphysiol.00530.2012. Epub 2012 Jun 28. J Appl Physiol (1985). 2012. PMID: 22744967 Free PMC article. - Effects of aging, exercise, and disease on force transfer in skeletal muscle.
Hughes DC, Wallace MA, Baar K. Hughes DC, et al. Am J Physiol Endocrinol Metab. 2015 Jul 1;309(1):E1-E10. doi: 10.1152/ajpendo.00095.2015. Epub 2015 May 12. Am J Physiol Endocrinol Metab. 2015. PMID: 25968577 Free PMC article. Review. - Pathways of Ca²⁺ entry and cytoskeletal damage following eccentric contractions in mouse skeletal muscle.
Zhang BT, Whitehead NP, Gervasio OL, Reardon TF, Vale M, Fatkin D, Dietrich A, Yeung EW, Allen DG. Zhang BT, et al. J Appl Physiol (1985). 2012 Jun;112(12):2077-86. doi: 10.1152/japplphysiol.00770.2011. Epub 2012 Mar 29. J Appl Physiol (1985). 2012. PMID: 22461447 Free PMC article. - Cycles of myofiber degeneration and regeneration lead to remodeling of the neuromuscular junction in two mammalian models of Duchenne muscular dystrophy.
Haddix SG, Lee YI, Kornegay JN, Thompson WJ. Haddix SG, et al. PLoS One. 2018 Oct 31;13(10):e0205926. doi: 10.1371/journal.pone.0205926. eCollection 2018. PLoS One. 2018. PMID: 30379896 Free PMC article. - Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner.
Dessem D, Ambalavanar R, Evancho M, Moutanni A, Yallampalli C, Bai G. Dessem D, et al. Pain. 2010 May;149(2):284-295. doi: 10.1016/j.pain.2010.02.022. Epub 2010 Mar 5. Pain. 2010. PMID: 20207080 Free PMC article.
References
- Alderton JM, Steinhardt RA. How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. Trends Cardiovasc Med. 2000;10:268–272. - PubMed
- Armstrong SC, Latham CA, Shivell CL, Ganote CE. Ischemic loss of sarcolemmal dystrophin and spectrin: correlation with myocardial injury. J Mol Cell Cardiol. 2001;33:1165–1179. - PubMed
- Augier N, Leger J, Robert A, Pons F, Leger JJ, Mornet D. Proteolytic susceptibility of the central domain in chicken gizzard and skeletal muscle dystrophins. Biochim Biophys Acta. 1992;1138:297–304. - PubMed
- Badorff C, Berkely N, Mehrotra S, Talhouk JW, Rhoads RE, Knowlton KU. Enteroviral protease 2A directly cleaves dystrophin and is inhibited by a dystrophin-based substrate analogue. J Biol Chem. 2000;275:11191–11197. - PubMed
- Badorff C, Lee GH, Lamphear BJ, Martone ME, Campbell KP, Rhoads RE, Knowlton KU. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 HD047099/HD/NICHD NIH HHS/United States
- T32 AR007592/AR/NIAMS NIH HHS/United States
- K-01-HD-01165/HD/NICHD NIH HHS/United States
- T-32-AR-07592-06/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources