Recognition and repair of the cyclobutane thymine dimer, a major cause of skin cancers, by the human excision nuclease - PubMed (original) (raw)
. 2003 Oct 15;17(20):2539-51.
doi: 10.1101/gad.1131003. Epub 2003 Oct 1.
Affiliations
- PMID: 14522951
- PMCID: PMC218148
- DOI: 10.1101/gad.1131003
Recognition and repair of the cyclobutane thymine dimer, a major cause of skin cancers, by the human excision nuclease
Joyce T Reardon et al. Genes Dev. 2003.
Abstract
The cyclobutane thymine dimer is the major DNA lesion induced in human skin by sunlight and is a primary cause of skin cancer, the most prevalent form of cancer in the Northern Hemisphere. In humans, the only known cellular repair mechanism for eliminating the dimer from DNA is nucleotide excision repair. Yet the mechanism by which the dimer is recognized and removed by this repair system is not known. Here we demonstrate that the six-factor human excision nuclease recognizes and removes the dimer at a rate consistent with the in vivo rate of removal of this lesion, even though none of the six factors alone is capable of efficiently discriminating the dimer from undamaged DNA. We propose a recognition mechanism by which the low-specificity recognition factors, RPA, XPA, and XPC, act in a cooperative manner to locate the lesion and, aided by the kinetic proofreading provided by TFIIH, form a high-specificity complex at the damage site that initiates removal of thymine dimers at a physiologically relevant rate and specificity.
Figures
Figure 1.
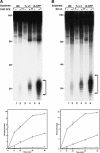
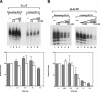
Removal of UV photoproducts by mammalian excision nuclease. (A) Excision by CHO cell-free extract (CFE). Duplexes of 136–140 bp (20 fmole) with no damage (UM, unmodified), with a cyclobutane thymine dimer (T<>T), or with a (6–4) photoproduct [(6–4)PP] were incubated with CHO CFE, and excision products were separated in 10% denaturing polyacrylamide gels. (Top) Autoradiogram of a representative assay; excision products are indicated by brackets to the right; numbers to the left indicate positions of DNA size markers. The reaction time was 60 min, and the excision levels as percent of substrate were 0.3% for UM DNA (lane 2), 1.8% for T<>T (lane 4), and 9.6% for (6–4)PP (lane 6). (Bottom) Excision kinetics. The averages of two experiments are plotted. (×) UM DNA; (▵) T<>T; (○) (6–4) photoproduct. (B) Excision with reconstituted human excision nuclease, RFI–VI (repair factors RPA, XPA, XPC, TFIIH, XPG, and XPF · ERCC1). (Top) Autoradiogram of a representative gel. Excision levels after 90 min of incubation were 0.1% for UM DNA (lane 2), 2.2% for T<>T (lane 4), and 16.4% for (6–4)PP (lane 6). (Bottom) Excision kinetics with RFI–VI. The averages of two to four experiments are plotted. (×) UM DNA; (▵) T<>T; (○) (6–4) photoproduct.
Figure 2.
Effect of photoreactivation on excision from the T<>T substrate. The substrate was mixed with photolyase (PL) and either kept in the dark or exposed to photoreactivating light as indicated. Then the DNA was extracted with phenol/chloroform, used in excision assays with CHO cell extracts, and separated in a 10% denaturing polyacrylamide gel. The numbers below the lanes indicate the average excision from two independent experiments.
Figure 3.
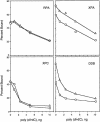
Binding of human damage recognition proteins to UV photoproducts. Electrophoretic mobility shift assays were done with the indicated proteins and 50-bp duplexes (0.4 nM) with no lesions (UM), with a T<>T, or with a (6–4) photoproduct as indicated. The lowest and highest protein concentrations in the assays are indicated by triangles above the autoradiograms, and the positions of free DNA are indicated to the left of each panel. At the right of each panel, binding isotherms generated from average values for three to five experiments are shown. (×) UM DNA; (▵) T<>T; (○) (6–4) photoproduct. For simplicity, only curves for UM and (6–4)PP DNA are shown.
Figure 4.
Binding of human damage recognition proteins to T<>T in the presence of competitive undamaged DNA. DNA substrates (5 fmole of 50-bp duplex) were mixed with indicated amounts of poly(dI · dC) and incubated with damage recognition proteins (concentrations in parentheses); then the fractions of bound radiolabeled probe were measured by electrophoretic mobility shift assays. The fractions bound in the absence of competitor were as follows. RPA (500 nM): 46.8% UM and 49.2% T<>T; XPA (200 nM): 58.1% UM and 73.2% T<>T; XPC (35 nM): 43.8% UM and 44.7% T<>T; DDB (3 nM): 42.9% UM and 70.1% T<>T. The average values for two to four independent experiments are plotted: (○) undamaged DNA; (▵) T<>T substrate.
Figure 5.
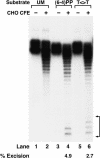
Excision of DNA damage from 50-bp duplex. Substrate DNA (20 fmole), with no lesion (UM), with a T<>T, or with a (6–4) photoproduct [(6–4)PP], was incubated with CHO cell extracts for 60 min, and products were separated in a 12% denaturing gel. Excision products are indicated by brackets to the right, and numbers below the lanes indicate excision levels as a percent of substrate DNA. With the 50-mer undamaged substrate no background excision was detected (lane 2).
Figure 6.
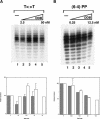
Effect of order-of-addition of repair factors on excision of T<>T. (A) Excision assays. Substrate (20 fmole) was preincubated with the indicated repair factor at 30°C for 10 min, then the remaining repair factors were added and incubation continued for the indicated times, and reaction products were separated in 10% denaturing polyacrylamide gels. The bracket indicates excision products. (B) Quantitative analysis of T<>T excision. The data points are averages for two to four independent experiments; for clarity, curve and error bars are shown only for the “no preincubation” reaction condition.
Figure 7.
Effect of DDB on excision of UV photoproducts by CHO cell-free extract. Substrates with a T<>T or (6–4) photoproduct (20 and 8 fmole, respectively) were incubated with the indicated amounts of DDB, and then CHO CFE with or without pBR322 was added and incubation continued for 90 min. (A) T<>T substrate. (Top) Excision gel (-pBR322) showing only the region encompassing the excision products; excision gels in the presence of pBR322 are not shown. (Bottom) Quantitative analysis of the data: (open bar) excision in the absence of pBR322; (closed bar) excision in the presence of pBR322. In the absence of DDB, the average excision in the absence or presence of pBR322 was 0.9% and 2.3%, respectively. Other values are expressed relative to these two values within each set. (B) The (6–4) photoproduct substrate. The gel and plot are as described in A. Without DDB, the average excision of input substrate in the absence and presence of pBR322 was 9.6% and 16.5%, respectively.
Figure 8.
Effect of DDB on excision of UV photoproducts by the six-factor human excision nuclease. T<>T and (6–4) photoproduct substrates (20 and 10 fmole, respectively) were incubated with DDB as indicated, and then RFI–VI were added and incubation was continued for another 90 min. (A) T<>T substrate. (Top) Excision gel showing only the region encompassing the excision products. The reactions in lanes 1_–_4 were carried out with optimal concentrations of RPA, XPA, XPC, and TFIIH; those in lanes 5_–_8 were performed with limiting concentrations of these repair factors. In the absence of DDB, the average excision values for optimal and limiting concentrations were 2.3% and 0.6%, respectively. (Bottom) Quantitative analysis of excision data from two to three experiments. (Closed bars) Optimal reaction conditions; (open bars) limiting recognition factors. Values are relative to the reaction with no DDB within each set, and standard errors are indicated. (B) The (6–4) photoproduct substrate. (Top) Excision gels; the average percent excision in the absence of DDB under optimal reaction conditions was 28.6% and under conditions of limiting recognition factors was 5.7%. (Bottom) Bar graph of averages for two to three experiments. (Closed bars) Optimal reaction conditions; (open bars) limiting recognition factors. Values are relative to the reaction with no DDB within each set, and standard errors are indicated.
Figure 9.
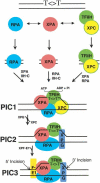
Model for human excision nuclease. Damage is recognized by RPA, XPA, and XPC in a “cooperative” manner, and the order of binding of these factors is “random.” Recruitment of TFIIH to form the four-factor preincision complex PIC1 and the ensuing helix unwinding provide an additional degree of specificity by “kinetic proofreading.” Further specificity is conferred by the preferential binding of XPG to the unwound DNA (PIC2). PIC3, a short-lived intermediate, is formed upon entry of XPF · ERCC1 and leads to dual incisions/excision.
Similar articles
- Thermodynamic cooperativity and kinetic proofreading in DNA damage recognition and repair.
Reardon JT, Sancar A. Reardon JT, et al. Cell Cycle. 2004 Feb;3(2):141-4. Cell Cycle. 2004. PMID: 14712076 Review. - Measurement of activities of cyclobutane-pyrimidine-dimer and (6-4)-photoproduct photolyases.
Hays JB, Hoffman P. Hays JB, et al. Methods Mol Biol. 1999;113:133-46. doi: 10.1385/1-59259-675-4:133. Methods Mol Biol. 1999. PMID: 10443416 No abstract available. - Model for XPC-independent transcription-coupled repair of pyrimidine dimers in humans.
Mu D, Sancar A. Mu D, et al. J Biol Chem. 1997 Mar 21;272(12):7570-3. doi: 10.1074/jbc.272.12.7570. J Biol Chem. 1997. PMID: 9065408 - Effects of microinjected photoreactivating enzyme on thymine dimer removal and DNA repair synthesis in normal human and xeroderma pigmentosum fibroblasts.
Roza L, Vermeulen W, Bergen Henegouwen JB, Eker AP, Jaspers NG, Lohman PH, Hoeijmakers JH. Roza L, et al. Cancer Res. 1990 Mar 15;50(6):1905-10. Cancer Res. 1990. PMID: 2306742 - Computational studies of DNA photolyase.
Harrison CB, O'Neil LL, Wiest O. Harrison CB, et al. J Phys Chem A. 2005 Aug 18;109(32):7001-12. doi: 10.1021/jp051075y. J Phys Chem A. 2005. PMID: 16834063 Review.
Cited by
- Dynamics of transcription-coupled repair of cyclobutane pyrimidine dimers and (6-4) photoproducts in Escherichia coli.
Adebali O, Sancar A, Selby CP. Adebali O, et al. Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2416877121. doi: 10.1073/pnas.2416877121. Epub 2024 Oct 23. Proc Natl Acad Sci U S A. 2024. PMID: 39441633 Free PMC article. - Does the XPA-FEN1 Interaction Concern to Nucleotide Excision Repair or Beyond?
Krasikova YS, Maltseva EA, Khodyreva SN, Evdokimov AN, Rechkunova NI, Lavrik OI. Krasikova YS, et al. Biomolecules. 2024 Jul 9;14(7):814. doi: 10.3390/biom14070814. Biomolecules. 2024. PMID: 39062528 Free PMC article. - Differing structures and dynamics of two photolesions portray verification differences by the human XPD helicase.
Fu I, Geacintov NE, Broyde S. Fu I, et al. Nucleic Acids Res. 2023 Dec 11;51(22):12261-12274. doi: 10.1093/nar/gkad974. Nucleic Acids Res. 2023. PMID: 37933861 Free PMC article. - Structural polymorphism of the PH domain in TFIIH.
Okuda M, Nishimura Y. Okuda M, et al. Biosci Rep. 2023 Jul 26;43(7):BSR20230846. doi: 10.1042/BSR20230846. Biosci Rep. 2023. PMID: 37340985 Free PMC article. - Nucleotide excision repair in Human cell lines lacking both XPC and CSB proteins.
Lindsey-Boltz LA, Yang Y, Kose C, Deger N, Eynullazada K, Kawara H, Sancar A. Lindsey-Boltz LA, et al. Nucleic Acids Res. 2023 Jul 7;51(12):6238-6245. doi: 10.1093/nar/gkad334. Nucleic Acids Res. 2023. PMID: 37144462 Free PMC article.
References
- Batty D., Rapic-Otrin, V., Levine, A.S., and Wood, R.D. 2000. Stable binding of human XPC complex to irradiated DNA confers strong discrimination for damaged sites. J. Mol. Biol. 300: 275-290. - PubMed
- Branum M.E., Reardon, J.T., and Sancar, A. 2001. DNA repair excision nuclease attacks undamaged DNA. J. Biol. Chem. 276: 25421-25426. - PubMed
- Brash D.E. 1997. Sunlight and the onset of skin cancer. Trends Genet. 13: 410-414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials