TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression - PubMed (original) (raw)
TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression
Binwu Tang et al. J Clin Invest. 2003 Oct.
Abstract
The TGF-beta signaling network plays a complex role in carcinogenesis because it has the potential to act as either a tumor suppressor or a pro-oncogenic pathway. Currently, it is not known whether TGF-beta can switch from tumor suppressor to pro-oncogenic factor during the course of carcinogenic progression in a single cell lineage with a defined initiating oncogenic event or whether the specific nature of the response is determined by cell type and molecular etiology. To address this question, we have introduced a dominant negative type II TGF-beta receptor into a series of genetically related human breast-derived cell lines representing different stages in the progression process. We show that decreased TGF-beta responsiveness alone cannot initiate tumorigenesis but that it can cooperate with an initiating oncogenic lesion to make a premalignant breast cell tumorigenic and a low-grade tumorigenic cell line histologically and proliferatively more aggressive. In a high-grade tumorigenic cell line, however, reduced TGF-beta responsiveness has no effect on primary tumorigenesis but significantly decreases metastasis. Our results demonstrate a causal role for loss of TGF-beta responsiveness in promoting breast cancer progression up to the stage of advanced, histologically aggressive, but nonmetastatic disease and suggest that at that point TGF-beta switches from tumor suppressor to prometastatic factor.
Figures
Figure 1
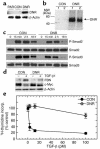
TGF-β receptor expression and responsiveness in the four human breast epithelial cell lines. (a) Ligand affinity cross-linking_._ Cell lines were affinity labeled with 100 pM 125I-labeled TGF-β1 in the absence (–) or presence (+) of a 50-fold molar excess of unlabeled TGF-β1. The migration positions of the endogenous TGF-β receptors (RI, RII, and RIII) are indicated. MW, molecular weight. (b) Smad phosphorylation and regulation of gene expression. Cells were treated with 2 ng/ml TGF-β and assessed by Western blot analysis for extent of Smad phosphorylation (at t =15 minutes), and effects on fibronectin (FBN) and c-Myc expression (at t = 24 hours). For the c-Myc blot, the arrow indicates the c-Myc band and the filled square indicates a nonspecific band. (c) Growth-inhibitory effects of TGF-β1. Cell proliferation in the presence of increasing amounts of TGF-β1 was quantitated by 3H-thymidine incorporation (incorp.). Results are the means for three determinations normalized to the controls with no added TGF-β for each cell type.
Figure 2
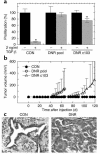
Blockade of TGF-β responses in vitro by transduction with the DNR. (a) Expression of the DNR in transduced cells. DNR expression in transduced M-III cells was determined by Western blot analysis probing for the Myc tag on the DNR. β-Actin protein was used for normalization. (b) Ability of the DNR to bind TGF-β. M-III cells were affinity labeled with 125I-TGF-β1. Following cross-linking, lysates were immunoprecipitated with anti-Myc Ab for visualization of ligand bound to the DNR. (c) Effect of DNR on Smad phosphorylation by TGF-β. M-III cells were treated with 5 ng/ml TGF-β1 for various times, and Smad protein expression and phosphorylation were analyzed by Western blot. P-Smad, phosphos-Smad. (d) Effect of DNR on gene-regulation responses to TGF-β1. M-III cells were treated with 5 ng/ml TGF-β1 or vehicle alone for 18 hours, and fibronectin (FBN) and c-Myc expression were analyzed by Western blot. (e) Effect of DNR on growth inhibition induced by TGF-β1. Growth inhibition in response to TGF-β1 was measured by [3H]-thymidine incorporation. All results are the mean ± SD for three determinations and are normalized to no TGF-β controls for each sample. PAR, untransduced parental M-III cells; CON, M-III cells transduced with pLPCX control retrovirus; DNR, M-III cells transduced with pLPC-DNR.
Figure 3
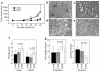
Decreased TGF-β responsiveness increases the probability of malignant conversion for the premalignant breast cell line M-II. (a) TGF-β responsiveness of M-II transductants in vitro. The proliferation of M-II transductants in the presence (+) and absence (–) of 2 ng/ml TGF-β1 was determined by incorporation of 3H-thymidine. Results are the mean ± SD of three determinations and are normalized to the no TGF-β control in each case. *P < 0.01. (b) Tumor growth kinetics. Five-week-old female athymic nude mice were inoculated subcutaneously on each hind flank with retrovirally transduced M-II cells (5 × 106 cells/site; ten sites per genotype). (c) Histology of lesions formed by M-II transductants. M-II CON cells formed cystic ductal structures, while MII-DNR formed glandular carcinomas. CON, M-II cells transduced with pLPCX; DNR-pool, pooled M-II cells transduced with pLPC-DNR at levels that fully blocked TGF-β growth-inhibitory responses; DNR-c103, a clone of M-II cells transduced with pLPC-DNR that retained partial TGF-β responsiveness.
Figure 4
Decreased TGF-β responsiveness increases the tumor growth rate and histological grade of the low-grade breast carcinoma line M-III. (a) Tumor growth kinetics. Nude mice were inoculated subcutaneously on each hind flank with retrovirally transduced M-III cells (106 cells/site; 10 sites/genotype). Untransduced parental M-III cells (n = 4, not shown) gave results essentially identical to M-III CON. (b–e). Histology of lesions formed by M-III transductants. Tumors of both genotypes were admixtures of three morphologic types. (b) M-III DNR tumor showing all three morphologies (Cr, cribriform structures; Cl, clear cells; At, area of atypia); (c) cribriform glands in an M-III CON tumor; (d) clear cell area in an M-III CON tumor; (e) area of atypia from an M-III DNR tumor. Magnification: ×100 (b) and ×400 (c–e). (f) Atypia and mitosis grades in M-III tumors. Histological sections were graded from 0 to 4 independently for extent of atypia and for frequency of mitoses as detailed in Methods. (g) Proliferation and apoptosis rates in M-III tumors. Tumor cell proliferation was quantitated by counting BrdU-labeled nuclei on histologic sections, and apoptotic cells were quantitated by TUNEL assay. Results are the mean ± SD for a minimum of five tumors of each genotype. PAR, parental untransduced cells; CON, cells transduced with pLPCX; DNR, cells transduced with pLPC-DNR. hpf, high-power field.
Figure 5
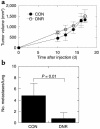
Decreased TGF-β responsiveness does not affect primary tumorigenesis but suppresses metastasis in the high-grade breast carcinoma line M-IV. (a) Tumor growth kinetics in vivo. Nude mice were inoculated subcutaneously on each hind flank with retrovirally transduced M-IV cells (2 × 105 cells/site). For M-IV CON, n = 11 sites injected; M-IV DNR, n = 11. Untransduced parental M-IV cells gave essential identical results to M-IV CON (n = 4, not shown). (b) Lung metastases. Metastatic efficiency was determined by quantitation of histologically detectable lung metastases 8 weeks after injection of 106 retrovirally transduced cells into the tail vein of nude mice. Results are the mean ± SD for n = 5 (M-IV CON) and n = 10 (M-IV DNR). CON, cells transduced with pLPCX; DNR, cells transduced with pLPC-DNR.
Figure 6
Summary of effect of decreased TGF-β response at different stages in the carcinogenic process for the breast.
Similar articles
- Smad-binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines.
Tian F, Byfield SD, Parks WT, Stuelten CH, Nemani D, Zhang YE, Roberts AB. Tian F, et al. Cancer Res. 2004 Jul 1;64(13):4523-30. doi: 10.1158/0008-5472.CAN-04-0030. Cancer Res. 2004. PMID: 15231662 - Transforming growth factor-beta in cancer and metastasis.
Jakowlew SB. Jakowlew SB. Cancer Metastasis Rev. 2006 Sep;25(3):435-57. doi: 10.1007/s10555-006-9006-2. Cancer Metastasis Rev. 2006. PMID: 16951986 Review. - Loss of responsiveness to transforming growth factor beta induces malignant transformation of nontumorigenic rat prostate epithelial cells.
Tang B, de Castro K, Barnes HE, Parks WT, Stewart L, Böttinger EP, Danielpour D, Wakefield LM. Tang B, et al. Cancer Res. 1999 Oct 1;59(19):4834-42. Cancer Res. 1999. PMID: 10519393 - Dissecting the role of TGF-beta type I receptor/ALK5 in pancreatic ductal adenocarcinoma: Smad activation is crucial for both the tumor suppressive and prometastatic function.
Schniewind B, Groth S, Sebens Müerköster S, Sipos B, Schäfer H, Kalthoff H, Fändrich F, Ungefroren H. Schniewind B, et al. Oncogene. 2007 Jul 19;26(33):4850-62. doi: 10.1038/sj.onc.1210272. Epub 2007 Feb 12. Oncogene. 2007. PMID: 17297450 - Duel nature of TGF-beta signaling: tumor suppressor vs. tumor promoter.
Bachman KE, Park BH. Bachman KE, et al. Curr Opin Oncol. 2005 Jan;17(1):49-54. doi: 10.1097/01.cco.0000143682.45316.ae. Curr Opin Oncol. 2005. PMID: 15608513 Review.
Cited by
- TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology.
Morikawa M, Derynck R, Miyazono K. Morikawa M, et al. Cold Spring Harb Perspect Biol. 2016 May 2;8(5):a021873. doi: 10.1101/cshperspect.a021873. Cold Spring Harb Perspect Biol. 2016. PMID: 27141051 Free PMC article. Review. - Cross-species genomic and functional analyses identify a combination therapy using a CHK1 inhibitor and a ribonucleotide reductase inhibitor to treat triple-negative breast cancer.
Bennett CN, Tomlinson CC, Michalowski AM, Chu IM, Luger D, Mittereder LR, Aprelikova O, Shou J, Piwinica-Worms H, Caplen NJ, Hollingshead MG, Green JE. Bennett CN, et al. Breast Cancer Res. 2012 Jul 19;14(4):R109. doi: 10.1186/bcr3230. Breast Cancer Res. 2012. PMID: 22812567 Free PMC article. - Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor beta in a mouse model of breast cancer.
Nam JS, Suchar AM, Kang MJ, Stuelten CH, Tang B, Michalowska AM, Fisher LW, Fedarko NS, Jain A, Pinkas J, Lonning S, Wakefield LM. Nam JS, et al. Cancer Res. 2006 Jun 15;66(12):6327-35. doi: 10.1158/0008-5472.CAN-06-0068. Cancer Res. 2006. PMID: 16778210 Free PMC article. - Disabled-2 downregulation promotes epithelial-to-mesenchymal transition.
Martin JC, Herbert BS, Hocevar BA. Martin JC, et al. Br J Cancer. 2010 Nov 23;103(11):1716-23. doi: 10.1038/sj.bjc.6605975. Epub 2010 Nov 9. Br J Cancer. 2010. PMID: 21063401 Free PMC article. - Actin Cytoskeleton and Focal Adhesions Regulate the Biased Migration of Breast Cancer Cells on Nanoscale Asymmetric Sawteeth.
Chen S, Hourwitz MJ, Campanello L, Fourkas JT, Losert W, Parent CA. Chen S, et al. ACS Nano. 2019 Feb 26;13(2):1454-1468. doi: 10.1021/acsnano.8b07140. Epub 2019 Feb 6. ACS Nano. 2019. PMID: 30707556 Free PMC article.
References
- Roberts, A.B., and Sporn, M.B. 1990. The transforming growth factors-β. In Handbook of experimental pharmacology. Peptide growth factors and their receptors. M.B. Sporn and A.B. Roberts, editors. Springer-Verlag. Berlin, Germany. 419–472.
- Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat. Genet. 2001;29:117–129. - PubMed
- Wakefield LM, Roberts AB. TGF-β signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 2002;12:22–29. - PubMed
- Massague J, Blain SW, Lo RS. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. - PubMed
- Fynan TM, Reiss M. Resistance to inhibition of cell growth by transforming growth factor-β and its role in oncogenesis. Crit. Rev. Oncog. 1993;4:493–540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials