In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life - PubMed (original) (raw)
. 2003 Oct 1;23(26):8844-53.
doi: 10.1523/JNEUROSCI.23-26-08844.2003.
Patrick C May, Mark A O'Dell, Jennie W Taylor, Maia Parsadanian, Jeffrey W Cramer, James E Audia, Jeffrey S Nissen, Kelly R Bales, Steven M Paul, Ronald B DeMattos, David M Holtzman
Affiliations
- PMID: 14523085
- PMCID: PMC6740389
- DOI: 10.1523/JNEUROSCI.23-26-08844.2003
In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life
John R Cirrito et al. J Neurosci. 2003.
Abstract
Soluble amyloid-beta (Abeta) peptide converts to structures with high beta-sheet content in Alzheimer's disease (AD). Soluble Abeta is released by neurons into the brain interstitial fluid (ISF), in which it can convert into toxic aggregates. Because assessment of ISF Abeta levels may provide unique insights into Abeta metabolism and AD, an in vivo microdialysis technique was developed to measure it. Our Abeta microdialysis technique was validated ex vivo with human CSF and then in vivo in awake, freely moving mice. Using human amyloid precursor protein (APP) transgenic mice, we found that, before the onset of AD-like pathology, ISF Abeta in hippocampus and cortex correlated with levels of APP in those tissues. After the onset of Abeta deposition, significant changes in the ISF Abeta40/Abeta42 ratio developed without changes in Abeta1-x. These changes differed from changes seen in tissue lysates from the same animals. By rapidly inhibiting Abeta production, we found that ISF Abeta half-life was short ( approximately 2 hr) in young mice but was twofold longer in mice with Abeta deposits. This increase in half-life, without an increase in steady-state levels, suggests that inhibition of Abeta synthesis reveals a portion of the insoluble Abeta pool that is in dynamic equilibrium with ISF Abeta. This now measurable in vivo pool is a likely target for new diagnostic and therapeutic strategies.
Figures
Figure 2.
Aβ ELISA and in vivo microdialysis technique. A, Aβ species detected by ELISA for human Aβ1-x, Aβ40, and Aβ42. Top, Aβ peptide sequence with epitopes recognized by each monoclonal (m) antibody. Bottom, Aβ species detected by each ELISA assay; solid lines represent segments of peptide that must be present for detection, and dashed lines represent segments of peptide that may vary but will still be recognized by the various antibody combinations. B, Awake mouse implanted with dual microdialysis probes. Black arrow denotes guide cannula-probe assembly; white arrow denotes collar used to attach mouse to balance arm that prevents force from being applied to implanted assembly. C, D, Representative probe placements in the hippocampus and striatum, respectively. Note partial probe tracts within each section. Hashed line depicts probe location. D, Cresyl violet stain of tissue surrounding the microdialysis probe tract after 18 hr implantation. There is no morphological evidence of substantial gliosis or inflammation along the tract (arrows), nor is there evidence of neuronal degeneration within the dentate granule cells. F, In vivo percentage recovery at various flow rates as determined by the interpolated zero flow method. At 0.5 and 2.6 μl/min, the percentage recovery of eAβ is 56.3 ± 4.21 and 4.9 ± 0.90% (n = 4), respectively.
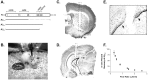
Figure 1.
In vitro microdialysis to measure Aβ. A, Diagram of exchangeable Aβ. Triangles represent potential Aβ binding molecules, e.g., apoE, clusterin, and α2M. Only highlighted Aβ molecules are of the appropriate size to pass through a 35 kDA MWCO membrane on the microdialysis probe. B, Interpolated zero flow method to quantify the pool of measurable Aβ1-x and Aβ40 within samples of human CSF (n = 4). At 2.2 μl/min, the percentage recovery of eAβ was 9.74 ± 1.53% (mean ± SEM). C, In vitro percentage recoveries for each Aβ species using the interpolated zero flow method. Each recovery point contains error bars and are overlapping for each species (n = 4). In vitro recovery of each Aβ species by microdialysis is the same. D, The concentration of eAβ and total soluble Aβ are highly correlated within a sample of human CSF (Pearson's r = 0.9487; p < 0.0001; n = 9). The mean concentrations of soluble Aβ1-x and eAβ1-x were 30.47 ± 4.23 ng/ml (mean ± SEM) and 196.2 ± 28.65pg/ml, respectively. E, F, Human CSF immunoprecipitated (IP'd) for Aβ has undetectable levels of total soluble Aβ and eAβ. Human CSF spiked with an amount of exogenous Aβ40 peptide expected to double Aβ concentration resulted in a 2.1-fold increase in total soluble Aβ and a 1.9-fold increase in eAβ.
Figure 3.
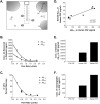
In vivo concentration of eAβ in young and middle-aged PDAPP mice. A, ISF eAβ concentrations in hippocampus and striatum of 3- and 12- to 15-month-old PDAPP mice. Three-month-old mice have 727.7 ± 92.1 pg/ml (n = 21; mean ± SEM) and 207.9 ± 31.8 pg/ml (n = 6) of eAβ1-x in the hippocampus and striatum, respectively. Twelve- to 15-month-old mice have 626.4 ± 93.3 pg/ml (n = 21) and 142.1 ± 19.5 pg/ml (n = 13) of eAβ in those regions. B, The concentration of eAβ species in young and aged mice. The concentration of eAβ1-x does not change significantly with age. eAβ40 increases significantly in the hippocampus with age (p = 0.0429), whereas eAβ42 does not decrease (p = 0.6116). C, The concentration of eAβ1-x within the hippocampus and striatum are highly correlated (Pearson's r = 0.7202; p < 0.001; n = 19). D, A microdialysis sample was analyzed by acid- urea electrophoresis, followed by Western blot analysis to visualize the various Aβ species present. On the basis of the peptide standard curves, Aβ42,Aβ40, and Aβ38 represent 15.6, 31.6, and 52.8%, respectively, of the ISF Aβ detected. No other Aβ species were detected. This confirms that Aβ-CTV is a major pool of Aβ in the ISF and that Aβ38 is the predominant species. E, The concentration of endogenous murine ISF eAβ40 and eAβ42 was measured in 3-month-old (n = 6) and 12-month-old (n = 6) C57BL/6 mice. There was no change with age in the concentration of murine eAβ40 (p = 0.718) or eAβ42 (p = 0.609) in wild-type mice.
Figure 4.
Differences in Aβ40/Aβ42 ratios between ISF, CSF, and brain tissue with age. In both the ISF and CSF fluids, the Aβ40/Aβ42 ratio increases significantly with age (p = 0.0466, n = 10; and p = 0.0413, n = 9, respectively), primarily attributable to an increase in Aβ40 within ISF and a decrease in Aβ42 in CSF. In contrast, carbonate (Carb) and guanidine (Guan) of hippocampal tissue reveals a significant decrease in the Aβ40/Aβ42 ratio with age (p < 0.0001 and p < 0.001, respectively; n = 10). This change coincides with a tremendous deposition of Aβ42 in older mice. Inset, Enlargement of hippocampal tissue lysate data.
Figure 5.
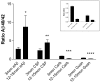
Relationship of Aβ between CNS compartments. A, There is no correlation between CSF Aβ and hippocampal (Hippo.) ISF eAβ in 3-month-old PDAPP mice (Pearson's r = 0.2192; p = 0.3530; n = 20). B, In 12- to 15-month-old PDAPP mice, there is a significant correlation between CSF Aβ and hippocampal ISF eAβ (Pearson's r = 0.6226; p = 0.023; n = 13). In middle-aged mice, there is a significant correlation between CSF Aβ and percentage Aβ load (C) (Pearson's r = 0.6641; p = 0.0133; n = 13), as well as a positive trend between ISF eAβ and percentage Aβ load in the hippocampus (D) (Pearson's r = 0.3511; p = 0.1582; n = 22).
Figure 6.
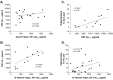
Half-life of ISF eAβ in young and middle-aged PDAPP mice. A, Effect of LY411575 on hippocampal eAβ1-x concentration in 3- and 12-month-old PDAPP mice. During microdialysis, animals received a 3 mg/kg subcutaneous injection of LY411575 or vehicle (corn oil). Concentrations of eAβ1-x dropped to 21.16 ± 11.46% (mean ± SEM; n = 4) and 22.34 ± 6.23% (n = 5) of baseline by 4 and 8 hr in young and middle-aged mice, respectively (p = 0.0045; repeated-measures ANOVA with Huynh and Feldt adjustment). Three-month-old (n = 5) and 12-month-old (n = 4) vehicle-injected mice do not show a significant change in Aβ concentration after treatment. B, A semi-log plot of percentage baseline of eAβ versus time is linear (young, y = -0.229_x_ + 2.11, _r_2 = 0.936; middle-aged, y = -0.099 + 2.10, _r_2 = 0.9173), suggesting first-order kinetics of Aβ elimination in both age groups. The half-life of Aβ within the ISF of young mice is 1.97 ± 0.623 hr (n = 4) and in aged mice is 3.78 ± 0.866 hr (n = 5). C, Concentration of LY411575 in whole brain of 3- and 12-month-old PDAPP mice 15 min after 3 mg/kg subcutaneous injection (n = 6). LY411575 enters the brain equally in both age groups with a concentration that was ∼220-fold higher than the IC50 needed to inhibit γ-secretase activity in cell culture. D, A Western blot probed for full-length and C-terminal fragments of APP. Tissue samples are from 3-month-old PDAPP mice injected with 3 mg/kg LY411575 or vehicle and killed at 8 hr after treatment (n = 8). Full-length APP levels do not change between these groups (top panel); however, both α-CTF and β-CTF increased in LY411575-treated mice (bottom panel), as expected with γ-secretase cleavage inhibited.
Figure 7.
Proposed diagram of Aβ metabolism within the CNS in the presence and absence of Aβ deposits. Aβ appears to be cleared from the ISF by efflux out of the brain and by local degradation (1), as well as by ISF to CSF bulk flow and ISF to periphery bulk flow (2). 3, Under normal conditions in the presence of deposits, the on-rate of Aβ into plaques is slow but faster than the off-rate, causing an accumulation onto Aβ plaques. 4, In the presence of a γ-secretase inhibitor, a fraction of the insoluble docked Aβ can become soluble and reenter the interstitial fluid.
Similar articles
- Dynamic analysis of amyloid β-protein in behaving mice reveals opposing changes in ISF versus parenchymal Aβ during age-related plaque formation.
Hong S, Quintero-Monzon O, Ostaszewski BL, Podlisny DR, Cavanaugh WT, Yang T, Holtzman DM, Cirrito JR, Selkoe DJ. Hong S, et al. J Neurosci. 2011 Nov 2;31(44):15861-9. doi: 10.1523/JNEUROSCI.3272-11.2011. J Neurosci. 2011. PMID: 22049429 Free PMC article. - Acute Effects of Muscarinic M1 Receptor Modulation on AβPP Metabolism and Amyloid-β Levels in vivo: A Microdialysis Study.
Welt T, Kulic L, Hoey SE, McAfoose J, Späni C, Chadha AS, Fisher A, Nitsch RM. Welt T, et al. J Alzheimers Dis. 2015;46(4):971-82. doi: 10.3233/JAD-150152. J Alzheimers Dis. 2015. PMID: 25881909 - Brain interstitial oligomeric amyloid β increases with age and is resistant to clearance from brain in a mouse model of Alzheimer's disease.
Takeda S, Hashimoto T, Roe AD, Hori Y, Spires-Jones TL, Hyman BT. Takeda S, et al. FASEB J. 2013 Aug;27(8):3239-48. doi: 10.1096/fj.13-229666. Epub 2013 May 2. FASEB J. 2013. PMID: 23640054 Free PMC article. - Intracellular biology of Alzheimer's disease amyloid beta peptide.
Hartmann T. Hartmann T. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):291-8. doi: 10.1007/s004060050102. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653285 Review. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
- In vivo hippocampal microdialysis reveals impairment of NMDA receptor-cGMP signaling in APP(SW) and APP(SW)/PS1(L166P) Alzheimer's transgenic mice.
Duszczyk M, Kuszczyk M, Guridi M, Lazarewicz JW, Sadowski MJ. Duszczyk M, et al. Neurochem Int. 2012 Dec;61(7):976-80. doi: 10.1016/j.neuint.2012.07.017. Epub 2012 Jul 27. Neurochem Int. 2012. PMID: 22841892 Free PMC article. - Rapid in vivo measurement of β-amyloid reveals biphasic clearance kinetics in an Alzheimer's mouse model.
Yuede CM, Lee H, Restivo JL, Davis TA, Hettinger JC, Wallace CE, Young KL, Hayne MR, Bu G, Li CZ, Cirrito JR. Yuede CM, et al. J Exp Med. 2016 May 2;213(5):677-85. doi: 10.1084/jem.20151428. Epub 2016 Apr 11. J Exp Med. 2016. PMID: 27069115 Free PMC article. - Systematic analysis of time-dependent neural effects of soluble amyloid β oligomers in culture and in vivo: Prevention by scyllo-inositol.
Jin M, Selkoe DJ. Jin M, et al. Neurobiol Dis. 2015 Oct;82:152-163. doi: 10.1016/j.nbd.2015.05.020. Epub 2015 Jun 6. Neurobiol Dis. 2015. PMID: 26054438 Free PMC article. - Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice.
Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, Gil SC, Brown J, Wilhelmsson U, Restivo JL, Cirrito JR, Holtzman DM, Kim J, Pekny M, Lee JM. Kraft AW, et al. FASEB J. 2013 Jan;27(1):187-98. doi: 10.1096/fj.12-208660. Epub 2012 Oct 4. FASEB J. 2013. PMID: 23038755 Free PMC article. - Low-density lipoprotein receptor-related protein 1: a physiological Aβ homeostatic mechanism with multiple therapeutic opportunities.
Sagare AP, Deane R, Zlokovic BV. Sagare AP, et al. Pharmacol Ther. 2012 Oct;136(1):94-105. doi: 10.1016/j.pharmthera.2012.07.008. Epub 2012 Jul 20. Pharmacol Ther. 2012. PMID: 22820095 Free PMC article. Review.
References
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM ( 1997) Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet 17: 263-264. - PubMed
- Benveniste H ( 1989) Brain microdialysis. J Neurochem 52: 1667-1679. - PubMed
- Benveniste H, Huttemeier PC ( 1990) Microdialysis—theory and application. Prog Neurobiol 35: 195-215. - PubMed
- Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI ( 2001) Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30: 665-676. - PubMed
- Cserr HF, Harling-Berg CJ, Knopf PM ( 1992) Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol 2: 269-276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG020222/AG/NIA NIH HHS/United States
- AG13956/AG/NIA NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- P01 AG011355/AG/NIA NIH HHS/United States
- M01 RR00036/RR/NCRR NIH HHS/United States
- R01 AG013956/AG/NIA NIH HHS/United States
- AG20222/AG/NIA NIH HHS/United States
- R37 AG013956/AG/NIA NIH HHS/United States
- AG11355/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical