A molecular mechanism for mRNA trafficking in neuronal dendrites - PubMed (original) (raw)
A molecular mechanism for mRNA trafficking in neuronal dendrites
Jianguo Shan et al. J Neurosci. 2003.
Abstract
Specific neuronal mRNAs are localized in dendrites, often concentrated in dendritic spines and spine synapses, where they are translated. The molecular mechanism of localization is mostly unknown. Here we have explored the roles of A2 response element (A2RE), a cis-acting signal for oligodendrocyte RNA trafficking, and its cognate trans-acting factor, heterogeneous nuclear ribonucleoprotein (hnRNP) A2, in neurons. Fluorescently labeled chimeric RNAs containing A2RE were microinjected into hippocampal neurons, and RNA transport followed using confocal laser scanning microscopy. These RNA molecules, but not RNA lacking the A2RE sequence, were transported in granules to the distal neurites. hnRNP A2 protein was implicated as the cognate trans-acting factor: it was colocalized with RNA in cytoplasmic granules, and RNA trafficking in neurites was compromised by A2RE mutations that abrogate hnRNP A2 binding. Coinjection of antibodies to hnRNP A2 halved the number of trafficking cells, and treatment of neurons with antisense oligonucleotides also disrupted A2RE-RNA transport. Colchicine inhibited trafficking, whereas cells treated with cytochalasin were unaffected, implicating involvement of microtubules rather than microfilaments. A2RE-like sequences are found in a subset of dendritically localized mRNAs, which, together with these results, suggests that a molecular mechanism based on this cis-acting sequence may contribute to dendritic RNA localization.
Figures
Figure 1.
Location of hnRNP A2 in rat hippocampal neurons. A, Confocal microscope image of hnRNP A2 in the nuclei (N) and neurites (arrows) of fixed hippocampal neurons. The protein was detected using a mouse primary antibody and an FITC-labeled goat anti-mouse secondary antibody. The endogenous hnRNP A2 is visible in granules distributed throughout the neurites. The inset at right is a twofold magnification of the region boxed at left. Scale bar, 15 μm. B, A control in which purified recombinant hnRNP A2 was added to the primary antibody before it was used for immunodetection of hnRNP A2 in neurons. Scale bar, 15 μm.
Figure 2.
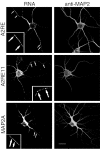
Transport of RNAs containing A2RE and A2RE-like elements in hippocampal neurons. Images of neurons in primary culture microinjected with fluorescently tagged RNA. After allowing 30 min after injection for RNA transport, the cells were fixed and stained for MAP2 protein to define the cell morphology. Dual-channel confocal microscopy was used to visualize the RNA (left panels) and protein (right panels). Each of the injected RNAs contained either A2RE or an A2RE-like element in the 3′UTR. RNAs bearing A2RE, A2RE11, or MAP2A elements were detected in granules in the soma and throughout the neurites (arrows). The insets show magnified regions. Scale bar, 15 μm.
Figure 3.
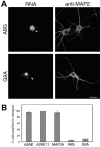
Mutations within the A2RE abolish trafficking in neurites. A, RNAs bearing the mutated elements A8G and G9A, which do not bind hnRNP A2, were observed only in the soma and proximal neurites (left panels, arrowheads). MAP2 was detected using mouse antibody and a tetramethylrhodamine isothiocyanate (TRITC)-labeled goat anti-mouse secondary antibody (right panels). Scale bar, 15 μm. B, Percentage of transport-positive cells for each of the exogenous RNAs in A (this Figure) and Figure 2. For each RNA, at least 40 cells were imaged for analysis. The error bars represent the SDs derived from the binomial distribution.
Figure 4.
Transport of A2RE-containing RNAs visualized in live neurons. Hippocampal neurons were microinjected with fluorescently labeled RNA (green) and Texas Red-labeled 10 kDa dextran (red), and after 30 min their distribution was determined by dual-channel confocal microscopy. A, Images of neurons microinjected with RNA. Exogenous RNAs containing A2RE or A2RE11 were observed to assemble into granules and move along the processes (arrows), whereas the A8G RNA did not assemble into granules but was distributed diffusely in the soma and proximal neurites (arrowhead) with only an occasional granule visible in the processes (arrows). Scale bar, 10 μm. B, Percentage of transport-positive cells for each of the microinjected RNAs in A. Error bars represent the SDs. For A2RE and A8G RNA, >20 cells were imaged for analysis. No error bar is given for A2RE11 because the number of cells imaged was small.
Figure 5.
Inhibition of RNA trafficking by antibodies to hnRNP A2. A2RE-containing RNA was microinjected into cultured neurons either without (top) or with (bottom) antibody. The cell morphology is shown by the distribution of Texas Red-conjugated dextran (left). The RNA is labeled with Alexa Fluor-UTP (right) as described in Materials and Methods, and the images are from live cells. Scale bar, 15 μm.
Figure 6.
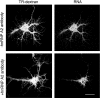
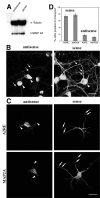
Effects of antisense oligonucleotide treatment on hnRNP A2 distribution and trafficking of A2RE-containing RNA. A, Western blot of hnRNP A2 in sense and antisense oligonucleotide-treated neurons with α-tubulin used as control for protein loading on the gel. B, Intracellular distribution of hnRNP A2 in hippocampal neurons after hnRNP A2 sense and antisense treatment. In sense-treated cells, the protein is observed in the soma (arrowhead) and as granules in the processes (arrows). In antisense-treated cells, the hnRNP A2 is confined primarily to the nucleus (arrowheads), with markedly lower levels in the processes (arrows). The same confocal microscopy data collection and image processing parameters were used for both samples. In both images the maximal brightness in the nucleus has been set just below 255, ensuring that all pixels are within the dynamic range of the imaging system, permitting direct comparison of the relative fluorescence intensities in the neurites in the two images. C, Representative images of hippocampal neurons microinjected with fluorescently labeled RNAs containing A2RE or the A2RE-like element of MAP2A after hnRNP A2 sense and antisense treatment. Injected RNAs were detected 50 μm or more from the cell bodies in sense-treated cells (right panels, arrows). In antisense-treated cells, RNAs were observed only in the proximal processes (left panels, arrowheads). D, Percentage of transport-positive, sense- and antisense-treated cells injected with RNA containing A2RE or the cognate sequence from MAP2A RNA. Error bars represent the SDs. Scale bar, 15 μm.
Figure 7.
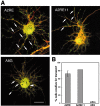
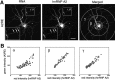
hnRNP A2 colocalization with injected A2RE-containing RNA in granules. Differentiated hippocampal neurons were microinjected with fluorescently labeled RNA. Mouse antibody to hnRNP A2 and TRITC-labeled secondary antibody were used to locate this protein after fixing the cells. The subcellular distributions of the injected RNA and hnRNP A2 were visualized and analyzed by dual-channel confocal microscopy. A, Confocal microscopy images of neurons microinjected with RNA containing A2RE. Insets, At left, higher magnifications of cell areas marked by squares at right to demonstrate the colocalization of hnRNP A2 and A2RE-containing RNAs (arrows). Scale bar, 15 μm. The merged images are shown at right, with the areas selected for fluorescence intensity measurements of granules indicated. B, Analysis of relative RNA- and hnRNP A2-associated fluorescence in individual granules. α, β, and γ indicate the regions in the merged images from A used for each of the analyses.
Figure 8.
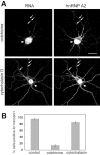
Effects of disruption of microtubules on A2RE RNA trafficking. A, The subcellular distribution of microinjected A2RE RNA (left panels) and of endogenous hnRNP A2 (right panels) was analyzed by dual-channel confocal microscopy in cells treated with either colchicine to disrupt microtubules (top panels) or cytochalasin to disrupt microfilaments (bottom panels). hnRNP A2 was detected in granules in the neurites of colchicine-treated cells, but these granules did not contain microinjected RNA (arrows). Scale bar, 15 μm. B, Percentage of transport-positive cells after treatment with colchicine or cytochalasin. In the control experiment, A2RE RNA was injected into untreated cells. Error bars represent the SDs.
Similar articles
- Heterogeneous nuclear ribonucleoprotein (hnRNP) E1 binds to hnRNP A2 and inhibits translation of A2 response element mRNAs.
Kosturko LD, Maggipinto MJ, Korza G, Lee JW, Carson JH, Barbarese E. Kosturko LD, et al. Mol Biol Cell. 2006 Aug;17(8):3521-33. doi: 10.1091/mbc.e05-10-0946. Epub 2006 Jun 14. Mol Biol Cell. 2006. PMID: 16775011 Free PMC article. - Mutational analysis of a heterogeneous nuclear ribonucleoprotein A2 response element for RNA trafficking.
Munro TP, Magee RJ, Kidd GJ, Carson JH, Barbarese E, Smith LM, Smith R. Munro TP, et al. J Biol Chem. 1999 Nov 26;274(48):34389-95. doi: 10.1074/jbc.274.48.34389. J Biol Chem. 1999. PMID: 10567417 - In neurons, activity-dependent association of dendritically transported mRNA transcripts with the transacting factor CBF-A is mediated by A2RE/RTS elements.
Raju CS, Fukuda N, López-Iglesias C, Göritz C, Visa N, Percipalle P. Raju CS, et al. Mol Biol Cell. 2011 Jun 1;22(11):1864-77. doi: 10.1091/mbc.E10-11-0904. Epub 2011 Apr 6. Mol Biol Cell. 2011. PMID: 21471000 Free PMC article. - Systems analysis of RNA trafficking in neural cells.
Carson JH, Barbarese E. Carson JH, et al. Biol Cell. 2005 Jan;97(1):51-62. doi: 10.1042/BC20040083. Biol Cell. 2005. PMID: 15601257 Review. - Moving molecules: mRNA trafficking in Mammalian oligodendrocytes and neurons.
Smith R. Smith R. Neuroscientist. 2004 Dec;10(6):495-500. doi: 10.1177/1073858404266759. Neuroscientist. 2004. PMID: 15534035 Review.
Cited by
- Mechanistic insights into the basis of widespread RNA localization.
Chekulaeva M. Chekulaeva M. Nat Cell Biol. 2024 Jul;26(7):1037-1046. doi: 10.1038/s41556-024-01444-5. Epub 2024 Jul 2. Nat Cell Biol. 2024. PMID: 38956277 Review. - The interferon-regulated host factor hnRNPA0 modulates HIV-1 production by interference with LTR activity, mRNA trafficking, and programmed ribosomal frameshifting.
Roesmann F, Sertznig H, Klaassen K, Wilhelm A, Heininger D, Heß S, Elsner C, Marschalek R, Santiago ML, Esser S, Sutter K, Dittmer U, Widera M. Roesmann F, et al. J Virol. 2024 Jul 23;98(7):e0053424. doi: 10.1128/jvi.00534-24. Epub 2024 Jun 20. J Virol. 2024. PMID: 38899932 - The Roles of hnRNP Family in the Brain and Brain-Related Disorders.
Brandão-Teles C, Antunes ASLM, de Moraes Vrechi TA, Martins-de-Souza D. Brandão-Teles C, et al. Mol Neurobiol. 2024 Jun;61(6):3578-3595. doi: 10.1007/s12035-023-03747-4. Epub 2023 Nov 24. Mol Neurobiol. 2024. PMID: 37999871 Review. - Heterogeneous nuclear ribonucleoprotein hnRNPA2/B1 regulates the abundance of the copper-transporter ATP7A in an isoform-dependent manner.
McCann CJ, Hasan NM, Padilla-Benavides T, Roy S, Lutsenko S. McCann CJ, et al. Front Mol Biosci. 2022 Dec 5;9:1067490. doi: 10.3389/fmolb.2022.1067490. eCollection 2022. Front Mol Biosci. 2022. PMID: 36545508 Free PMC article. - Wiskott-Aldrich syndrome protein forms nuclear condensates and regulates alternative splicing.
Yuan B, Zhou X, Suzuki K, Ramos-Mandujano G, Wang M, Tehseen M, Cortés-Medina LV, Moresco JJ, Dunn S, Hernandez-Benitez R, Hishida T, Kim NY, Andijani MM, Bi C, Ku M, Takahashi Y, Xu J, Qiu J, Huang L, Benner C, Aizawa E, Qu J, Liu GH, Li Z, Yi F, Ghosheh Y, Shao C, Shokhirev M, Comoli P, Frassoni F, Yates JR 3rd, Fu XD, Esteban CR, Hamdan S, Izpisua Belmonte JC, Li M. Yuan B, et al. Nat Commun. 2022 Jun 25;13(1):3646. doi: 10.1038/s41467-022-31220-8. Nat Commun. 2022. PMID: 35752626 Free PMC article.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM ( 2001) Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30: 489-502. - PubMed
- Barbarese E, Koppel DE, Deutscher MP, Smith CL, Ainger K, Morgan F, Carson JH ( 1995) Protein translation components are colocalized in granules in oligodendrocytes. J Cell Sci 108: 2781-2790. - PubMed
- Bassell GJ, Oleynikov Y, Singer RH ( 1999) The travels of mRNAs through all cells large and small. FASEB J 13: 447-454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS015190/NS/NINDS NIH HHS/United States
- Wellcome Trust/United Kingdom
- R01 NS019943/NS/NINDS NIH HHS/United States
- R56 NS015190/NS/NINDS NIH HHS/United States
- NS15190/NS/NINDS NIH HHS/United States
- NS19943/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases