Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum - PubMed (original) (raw)
Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum
Jaime N Guzmán et al. J Neurosci. 2003.
Abstract
Dopamine is a critical modulator of striatal function; its absence produces Parkinson's disease. Most cellular actions of dopamine are still unknown. This work describes the presynaptic actions of dopaminergic receptor agonists on GABAergic transmission between neostriatal projection neurons. Axon collaterals interconnect projection neurons, the main axons of which project to other basal ganglia nuclei. Most if not all of these projecting axons pass through the globus pallidus. Thus, we lesioned the intrinsic neurons of the globus pallidus and stimulated neostriatal efferent axons antidromically with a bipolar electrode located in this nucleus. This maneuver revealed a bicuculline-sensitive synaptic current while recording in spiny cells. D1 receptor agonists facilitated whereas D2 receptor agonists depressed this synaptic current. In contrast, a bicuculline-sensitive synaptic current evoked by field stimulation inside the neostriatum was not consistently modulated, in agreement with previous studies. The data are discussed in light of the most recent experimental and modeling results. The conclusion was that inhibition of spiny cells by axon collaterals of other spiny cells is quantitatively important; however, to be functionally important, this inhibition might be conditioned to the synchronized firing of spiny neurons. Finally, dopamine exerts a potentially important role regulating the extent of lateral inhibition.
Figures
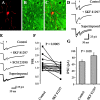
Figure 1.
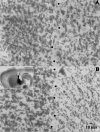
Pallidal lesions. A, A 40 μm histological section taken from a sagittal 300 μm slice obtained from an animal perfused transcardially with saline containing choline instead of Na+ (see Materials and Methods). Dots approximately depict the border between pallidum (right) and neostriatum (left). B, A similar section taken from an ibotenic acid-lesioned animal. Notice a marked reduction in cellular profiles in the right side (pallidum). The inset shows the approximate trajectory of the lesioning needle when using the coordinates described in Materials and Methods. Injections were placed as far as possible from the striatopallidal border to avoid diffusion of ibotenic acid into the neostriatum. Methylene blue was used to stain the injection site. Histological sections were processed for Nissl staining.
Figure 2.
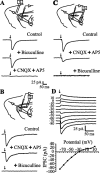
Orthodromic responses evoked antidromically. A, Top to bottom, Striatal population spike (1) voltage recording (2) and whole-cell currents (3) evoked during GP stimulation in the absence of CNQX or QX-314. B, The scheme depicts the experimental protocol to evoke synaptic activity with glutamate puffs (1). Synaptic activity is enhanced only after a glutamate puff in the neostriatum (arrows) (2). C, IPSCs evoked after antidromic stimulation of striatal axons in the GP. Weak stimulus strength produces paired-pulse facilitation (1), whereas stronger stimulus strength evokes paired-pulse depression (2). Twenty-five trials with failures were averaged for each trace. The intensity-amplitude relationship was plotted (3) for both first (filled circles) and second (filled squares) IPSCs. St, Striatum.
Figure 3.
Evoked synaptic responses in a corticostriatal slice preparation. Top scheme in each frame illustrates the position of stimulating and recording electrodes; two medium spiny (round) and one local interneuron (ovoid) are symbolized. A, Synaptic currents were evoked by cortical stimulation (in this case, recordings are averages of 200 trials). Currents were blocked by CNQX (10 μ
m
) plus AP5 (50 μ
m
). No bicuculline-sensitive component was recorded with these stimulating conditions (1-4 V) (see Materials and Methods). B, Stimulation and recording in the neostriatum evoked synaptic currents that were only partially blocked by glutamatergic antagonists (CNQX and AP5 as before). Bicuculline (10 μ
m
) blocked a GABAergic component. C, Stimulation in the GP (lesioned with ibotenic acid) and recording in the neostriatum. Axons from spiny cells were activated antidromically to turn on collaterals interconnecting spiny cells. Glutamatergic components (cortical projections also pass through GP) were blocked by CNQX plus AP5. A clear bicuculline-sensitive component was disclosed. D, Synaptic currents evoked by antidromic stimulation in the GP. Reversal potential was -28.7 ± 7 mV (n = 12), which was not significantly different from the chloride equilibrium potential (-30.5 mV).
Figure 8.
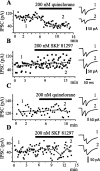
Time course of action of dopaminergic drugs. IPSCs exhibited amplitude variation from trial to trial, suggesting that only a few terminals were being activated. Amplitudes in each trial and mean amplitude of first IPSC were graphed in all cases (A-D). A, IPSCs were evoked with antidromic stimulation from the GP, thus favoring activation of terminals from recurrent axon collaterals (as in Fig. 1_C_). After several minutes of control recordings, 200 n
m
quinelorane (dopaminergic D2 receptor agonist) was added to the superfusion. Quinelorane reduced IPSC amplitude and produced paired-pulse facilitation (PPR >1). Recordings at right, in this and the other frames, were taken before and during drug application, as indicated by the numbers. B, Same experimental arrangement as in A, except that 200 n
m
SKF 81297 (dopaminergic D1 receptor agonist) was administered. A tendency to exhibit larger IPSC amplitudes is accompanied with paired-pulse depression (PPR <1). C, IPSCs are now intrastriatally evoked (as in Fig. 1 B). Quinelorane produced neither amplitude nor PPR changes in most cases. D, Same experimental arrangement as in C, except that SKF 81297 is administered. In this case, there was no consistent change of either mean amplitude or PPR. In several cases, the IPSC was reduced. Stimulus frequency was 0.2 Hz. The traces at right are averages of 2 min recordings at approximate times indicated by the numbers.
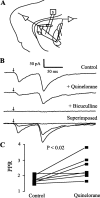
Figure 5.
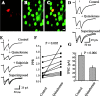
Dopaminergic modulation of medium spiny axon collaterals. Activation of D2 receptors. Stimulating and recording electrodes as in Figure 1_C_. A, Neostriatal neuron filled with biocytin. B, Same preparation showing neurons immunoreactive for SP. C, Superimposition of A and B with confocal microscopy; recorded neuron was SP positive. D, Top to bottom, First control synaptic current (in CNQX plus AP5) was reduced by 100 n
m
quinelorane, whereas PPR was increased. Bottom trace shows superimposition of top and middle traces. E, Sulpiride (200 n
m
) reverses the action of quinelorane (100 n
m
) in another cell. F, Paired line graph illustrates PPR changes in a sample of spiny neurons (p < 0.005; n = 10). G, Using the same stimulus strength, mean IPSC amplitude (first response of the pair) before and during quinelorane is significantly different (p < 0.006).
Figure 6.
Dopaminergic modulation of medium spiny axon collaterals. Activation of D1 receptors. Stimulating and recording electrodes as in Figure 1_C_. A, Neostriatal neuron filled with biocytin. B, Same preparation showing neurons immunoreactive for ENK. C, Superimposition of A and B with confocal microscopy; recorded neuron was ENK-positive. D, Top to bottom, First control synaptic current (in CNQX + AP5) was enhanced (12 of 16) by 100 n
m
SKF 21897, whereas PPR was decreased (14 of 16). Bottom trace shows superimposition of top and middle traces. E, SCH 23390 (100 n
m
) reverses the action of SKF 81297 in another cell. F, Paired line graph illustrates PPR reductions in a sample of spiny neurons (p < 0.0005; n = 16). G, Using the same stimulus strength, the mean of IPSC amplitudes (first response of the pair) before and during SKF 81297.
Figure 4.
Dopaminergic modulation of striatopallidal transmission. A, Recording of pallidal neurons during striatal stimulation. B, Top to bottom, Control synaptic currents in the presence of CNQX (10 μ
m
) and AP5 (50 μ
m
). The action of quinelorane (100 n
m
) reduced synaptic currents and increased PPR in six of seven cells; bicuculline blocked all currents evoked from the striatum. The last trace superimposes all of the above records. C, Graph summarizing the results.
Figure 7.
Lack of consistent dopaminergic modulation of synaptic currents evoked with intrastriatal field stimulation. Stimulating and recording electrodes as in Figure 1_B_. A, Quinelorane (100 n
m
) does not consistently change IPSC amplitude or PPR. B, Paired line graph shows an inconsistent pattern of PPR change. No more than one-third of cases exhibited a PPR increase (p > 0.4); some showed a decrease. C, When evoked intrastriatally, mean IPSC amplitude did not change during D2 agonist (p > 0.2). D, SKF 81297 (100 n
m
) does not consistently change PPR. In the case shown, IPSC was reduced (in contrast to GP antidromic stimulation). E, Paired line graph shows an irregular pattern of PPR change (p > 0.6). F, Intrastriatally evoked mean IPSC exhibited a striking contrast with antidromically evoked mean IPSC; its average amplitude is reduced instead of enhanced (p < 0.01).
Similar articles
- Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo.
Paladini CA, Celada P, Tepper JM. Paladini CA, et al. Neuroscience. 1999 Mar;89(3):799-812. doi: 10.1016/s0306-4522(98)00355-8. Neuroscience. 1999. PMID: 10199614 - Dopaminergic modulation of excitatory postsynaptic currents in rat neostriatal neurons.
Umemiya M, Raymond LA. Umemiya M, et al. J Neurophysiol. 1997 Sep;78(3):1248-55. doi: 10.1152/jn.1997.78.3.1248. J Neurophysiol. 1997. PMID: 9310416 - GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata.
Celada P, Paladini CA, Tepper JM. Celada P, et al. Neuroscience. 1999 Mar;89(3):813-25. doi: 10.1016/s0306-4522(98)00356-x. Neuroscience. 1999. PMID: 10199615 - GABAergic control of substantia nigra dopaminergic neurons.
Tepper JM, Lee CR. Tepper JM, et al. Prog Brain Res. 2007;160:189-208. doi: 10.1016/S0079-6123(06)60011-3. Prog Brain Res. 2007. PMID: 17499115 Review. - When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function.
Plenz D. Plenz D. Trends Neurosci. 2003 Aug;26(8):436-43. doi: 10.1016/S0166-2236(03)00196-6. Trends Neurosci. 2003. PMID: 12900175 Review.
Cited by
- The balance of striatal feedback transmission is disrupted in a model of parkinsonism.
López-Huerta VG, Carrillo-Reid L, Galarraga E, Tapia D, Fiordelisio T, Drucker-Colin R, Bargas J. López-Huerta VG, et al. J Neurosci. 2013 Mar 13;33(11):4964-75. doi: 10.1523/JNEUROSCI.4721-12.2013. J Neurosci. 2013. PMID: 23486967 Free PMC article. - Dopamine-deprived striatal GABAergic interneurons burst and generate repetitive gigantic IPSCs in medium spiny neurons.
Dehorter N, Guigoni C, Lopez C, Hirsch J, Eusebio A, Ben-Ari Y, Hammond C. Dehorter N, et al. J Neurosci. 2009 Jun 17;29(24):7776-87. doi: 10.1523/JNEUROSCI.1527-09.2009. J Neurosci. 2009. PMID: 19535589 Free PMC article. - Dopaminergic treatment weakens medium spiny neuron collateral inhibition in the parkinsonian striatum.
Wei W, Ding S, Zhou FM. Wei W, et al. J Neurophysiol. 2017 Mar 1;117(3):987-999. doi: 10.1152/jn.00683.2016. Epub 2016 Dec 7. J Neurophysiol. 2017. PMID: 27927785 Free PMC article. - Dopamine presynaptically and heterogeneously modulates nucleus accumbens medium-spiny neuron GABA synapses in vitro.
Geldwert D, Norris JM, Feldman IG, Schulman JJ, Joyce MP, Rayport S. Geldwert D, et al. BMC Neurosci. 2006 Jun 30;7:53. doi: 10.1186/1471-2202-7-53. BMC Neurosci. 2006. PMID: 16813648 Free PMC article. - MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants.
Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE. Deng JV, et al. Nat Neurosci. 2010 Sep;13(9):1128-36. doi: 10.1038/nn.2614. Epub 2010 Aug 15. Nat Neurosci. 2010. PMID: 20711186 Free PMC article.
References
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A ( 2000) Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci 3: 226-230. - PubMed
- Albin RL, Young AB, Penney JB ( 1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366-375. - PubMed
- Aronin N, Chase K, DiFiglia M ( 1986) Glutamic acid decarboxylase and enkephalin immunoreactive axon terminals in the rat neostriatum synapse with striatonigral neurons. Brain Res 365: 151-158. - PubMed
- Bar-Gad I, Bergman H ( 2001) Stepping out of the box: information processing in the neural networks of the basal ganglia. Curr Opin Neurobiol 11: 689-695. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources