Glutamate and amyloid beta-protein rapidly inhibit fast axonal transport in cultured rat hippocampal neurons by different mechanisms - PubMed (original) (raw)
Glutamate and amyloid beta-protein rapidly inhibit fast axonal transport in cultured rat hippocampal neurons by different mechanisms
Hiromi Hiruma et al. J Neurosci. 2003.
Abstract
Impairment of axonal transport leads to neurodegeneration and synapse loss. Glutamate and amyloid beta-protein (Abeta) have critical roles in the pathogenesis of Alzheimer's disease (AD). Here we show that both agents rapidly inhibit fast axonal transport in cultured rat hippocampal neurons. The effect of glutamate (100 microm), but not of Abeta25-35 (20 microm), was reversible, was mimicked by NMDA or AMPA, and was blocked by NMDA and AMPA antagonists and by removal of extracellular Ca2+. The effect of Abeta25-35 was progressive and irreversible, was prevented by the actin-depolymerizing agent latrunculin B, and was mimicked by the actin-polymerizing agent jasplakinolide. Abeta25-35 induced intracellular actin aggregation, which was prevented by latrunculin B. Abeta31-35 but not Abeta15-20 exerted effects similar to those of Abeta25-35. Full-length Abeta1-42 incubated for 7 d, which specifically contained 30-100 kDa molecular weight assemblies, also caused an inhibition of axonal transport associated with intracellular actin aggregation, whereas freshly dissolved Abeta1-40, incubated Abeta1-40, and fresh Abeta1-42 had no effect. These results suggest that glutamate inhibits axonal transport via activation of NMDA and AMPA receptors and Ca2+ influx, whereas Abeta exerts its inhibitory effect via actin polymerization and aggregation. The ability of Abeta to inhibit axonal transport seems to require active amino acid residues, which is probably present in the 31-35 sequence. Full-length Abeta may be effective when it represents a structure in which these active residues can access the cell membrane. Our results may provide insight into the early pathogenetic mechanisms of AD.
Figures
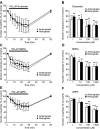
Figure 1.
Effects of glutamate and its agonists on the number of particles transported in neurites of cultured rat hippocampal neurons. A, C, E, Percentage changes in the number of transported particles of control (the value before application) induced by 26 min application of 100 μ
m
glutamate (A), 100 μ
m
NMDA (C), and 100 μ
m
AMPA (E). Note that the number of transported particles was rapidly reduced by these drugs and restored by removal of the drugs. B, D, F, Concentration dependence of reduction in the number of transported particles induced by glutamate (B), NMDA (D), and AMPA (F). The value obtained at 20 min after each application of the drugs is expressed as a percentage of control (the value before application). Data in all panels are expressed as mean ± SD of five neurons. Error bars represent SD. *p < 0.05; **p < 0.005; ***p < 0.0005 compared with control (100%).
Figure 3.
Morphological changes induced by glutamate and Aβ25-35 during video-enhanced microscopic recordings. Video images of neurites were acquired before (Control) and 20 min after treatment with 100 μ
m
glutamate (A), and before (Control) and 20 min after treatment with 20 μ
m
Aβ25-35 (B). Note that the membrane in the neurite was blebbing in glutamate-treated neurons, whereas the neurite was shrunken and microtubules were clearly visible in the Aβ25-35-treated neuron. Scale bar, 2 μm.
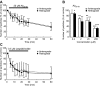
Figure 2.
Effects of Aβ25-35 and jasplakinolide, an actin-polymerizing agent, on the number of particles transported in neurites of cultured rat hippocampal neurons. A, C, Percentage changes in the number of transported particles of control (the value before application) induced by 26 min application of 20 μ
m
Aβ25-35 (A) and 0.5 μ
m
jasplakinolide (C). Note that the number of transported particles was rapidly and progressively reduced by both drugs and not restored after removal of the drugs. B, Concentration dependence of reduction in the number of transported particles induced by Aβ25-35. The value obtained at 20 min after each application of various concentrations of Aβ25-35 is expressed as a percentage of control (the value before application). Data in all panels are expressed as mean ± SD of five neurons. Error bars represent SD. *p < 0.05; **p < 0.005; ***p < 0.0005 compared with control (100%).
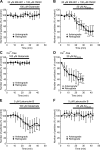
Figure 4.
Effects of glutamate and Aβ25-35 under various conditions. Data represent percentage changes in the number of transported particles of control (the value before application). Glutamate (100 μ
m
) (A, C, E) and Aβ25-35 (20 μ
m
) (B, D, F) were applied after treatment with a combination of 20 μ
m
MK-801, an NMDA receptor antagonist, and 100 μ
m
CNQX, an AMPA receptor antagonist (A, B), in Ca2+-free solution (C, D), and after treatment with 5 μ
m
latrunculin B, an actin depolymerizer (E, F). Each data point indicates the mean ± SD of the values obtained from five neurons. Error bars represent SD. *p < 0.05; **p < 0.005; ***p < 0.0005 compared with control (100%).
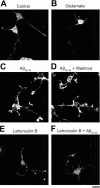
Figure 5.
Rhodamine-phalloidin staining of actin filaments in cultured hippocampal neurons. Neurons were stained with rhodamine-phalloidin after treatment of drugs dissolved in HEPES-buffered solution. A, Control (treatment with HEPES-buffered solution alone for 30 min). B, Treatment with 100 μ
m
glutamate for 30 min. C, Treatment with 20 μ
m
Aβ25-35 for 30 min. D, Treatment with 20 μ
m
Aβ25-35 for 30 min followed by washout for 30 min. E, Treatment with 5 μ
m
latrunculin B for 30 min. F, Simultaneous treatment with 20 μ
m
Aβ25-35 and 5 μ
m
latrunculin B for 30 min. Scale bar, 20 μm.
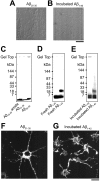
Figure 6.
Photographs representing properties of various Aβ fragments. A, B, Precipitates detected under light microscopy in Aβ25-35 solution (A) and in incubated Aβ1-40 solution (B). Aβ25-35 produced cotton-like precipitates (A), and incubated Aβ1-40 formed fiber-like or amorphous precipitates (B). Scale bar, 10 μm. C-E, SDS-PAGE profiles of various Aβ fragments. F, G, Rhodamine-phalloidin staining of intracellular actin filaments in hippocampal neurons treated with Aβ31-35 (F) and with incubated Aβ1-42 (G). Aβ31-35 (20 μ
m
) and incubated Aβ1-42 (20 μ
m
) formed intracellular actin aggregates. Scale bar, 20 μm.
Similar articles
- Neuropeptide Y release from cultured hippocampal neurons: stimulation by glutamate acting at N-methyl-D-aspartate and AMPA receptors.
Gemignani A, Marchese S, Fontana G, Raiteri M. Gemignani A, et al. Neuroscience. 1997 Nov;81(1):23-31. doi: 10.1016/s0306-4522(97)00168-1. Neuroscience. 1997. PMID: 9300398 - Soluble beta-amyloid1-40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses.
Roselli F, Tirard M, Lu J, Hutzler P, Lamberti P, Livrea P, Morabito M, Almeida OF. Roselli F, et al. J Neurosci. 2005 Nov 30;25(48):11061-70. doi: 10.1523/JNEUROSCI.3034-05.2005. J Neurosci. 2005. PMID: 16319306 Free PMC article. - Amyloid beta proteins inhibit Cl(-)-ATPase activity in cultured rat hippocampal neurons.
Yagyu K, Kitagawa K, Irie T, Wu B, Zeng XT, Hattori N, Inagaki C. Yagyu K, et al. J Neurochem. 2001 Aug;78(3):569-76. doi: 10.1046/j.1471-4159.2001.00446.x. J Neurochem. 2001. PMID: 11483660 - Alzheimer's amyloid-beta peptide inhibits sodium/calcium exchange measured in rat and human brain plasma membrane vesicles.
Wu A, Derrico CA, Hatem L, Colvin RA. Wu A, et al. Neuroscience. 1997 Oct;80(3):675-84. doi: 10.1016/s0306-4522(97)00053-5. Neuroscience. 1997. PMID: 9276485 - Susceptibility of hippocampal neurons to Abeta peptide toxicity is associated with perturbation of Ca2+ homeostasis.
Resende R, Pereira C, Agostinho P, Vieira AP, Malva JO, Oliveira CR. Resende R, et al. Brain Res. 2007 Apr 27;1143:11-21. doi: 10.1016/j.brainres.2007.01.071. Epub 2007 Jan 27. Brain Res. 2007. PMID: 17336275
Cited by
- Links between electrophysiological and molecular pathology of amyotrophic lateral sclerosis.
Quinlan KA. Quinlan KA. Integr Comp Biol. 2011 Dec;51(6):913-25. doi: 10.1093/icb/icr116. Epub 2011 Oct 11. Integr Comp Biol. 2011. PMID: 21989221 Free PMC article. Review. - Mapping cofilin-actin rods in stressed hippocampal slices and the role of cdc42 in amyloid-beta-induced rods.
Davis RC, Maloney MT, Minamide LS, Flynn KC, Stonebraker MA, Bamburg JR. Davis RC, et al. J Alzheimers Dis. 2009;18(1):35-50. doi: 10.3233/JAD-2009-1122. J Alzheimers Dis. 2009. PMID: 19542631 Free PMC article. - Glutamate excitotoxicity is involved in the induction of paralysis in mice after infection by a human coronavirus with a single point mutation in its spike protein.
Brison E, Jacomy H, Desforges M, Talbot PJ. Brison E, et al. J Virol. 2011 Dec;85(23):12464-73. doi: 10.1128/JVI.05576-11. Epub 2011 Sep 28. J Virol. 2011. PMID: 21957311 Free PMC article. - Tau reduction prevents Abeta-induced defects in axonal transport.
Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Vossel KA, et al. Science. 2010 Oct 8;330(6001):198. doi: 10.1126/science.1194653. Epub 2010 Sep 9. Science. 2010. PMID: 20829454 Free PMC article. - Enhanced β-secretase processing alters APP axonal transport and leads to axonal defects.
Rodrigues EM, Weissmiller AM, Goldstein LS. Rodrigues EM, et al. Hum Mol Genet. 2012 Nov 1;21(21):4587-601. doi: 10.1093/hmg/dds297. Epub 2012 Jul 27. Hum Mol Genet. 2012. PMID: 22843498 Free PMC article.
References
- Arias C, Becerra-García F, Tapia R ( 1998) Glutamic acid and Alzheimer's disease. Neurobiology 6: 33-43. - PubMed
- Bai R, Covell DG, Liu C, Ghosh AK, Hamel E ( 2002) (-)-Doliculide, a new macrocyclic depsipeptide enhancer of actin assembly. J Biol Chem 277: 32165-32171. - PubMed
- Barrow CJ, Zagorski MG ( 1991) Solution structures of β peptide and its constituent fragments: relation to amyloid deposition. Science 253: 179-182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous