Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae - PubMed (original) (raw)
Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae
Matthew H Bender et al. J Bacteriol. 2003 Oct.
Abstract
CpsA, CpsB, CpsC, and CpsD are part of a tyrosine phosphorylation regulatory system involved in modulation of capsule synthesis in Streptococcus pneumoniae and many other gram-positive and gram-negative bacteria. Using an immunoblotting technique, we observed distinct laddering patterns of S. pneumoniae capsular polysaccharides of various serotypes and found that transfer of the polymer from the membrane to the cell wall was independent of size. Deletion of cps2A, cps2B, cps2C, or cps2D in the serotype 2 strain D39 did not affect the ability to transfer capsule to the cell wall. Deletion of cps2C or cps2D, which encode two domains of an autophosphorylating tyrosine kinase, resulted in the production of only short-chain polymers. The function of Cps2A is unknown, and the polymer laddering pattern of the cps2A deletion mutants appeared similar to that of the parent, although the total amount of capsule was decreased. Loss of Cps2B, a tyrosine phosphatase and a kinase inhibitor, resulted in an increase in capsule amount and a normal ladder pattern. However, Cps2B mutants exhibited reduced virulence following intravenous inoculation of mice and were unable to colonize the nasopharynx, suggesting a diminished capacity to sense or respond to these environments. In D39 and its isogenic mutants, the amounts of capsule and tyrosine-phosphorylated Cps2D (Cps2D approximately P) correlated directly. In contrast, restoration of type 2 capsule production followed by deletion of cps2B in Rx1, a laboratory passaged D39 derivative containing multiple uncharacterized mutations, resulted in decreased capsule amounts but no alteration in Cps2D approximately P levels. Thus, a factor outside the capsule locus, which is either missing or defective in the Rx1 background, is important in the control of capsule synthesis.
Figures
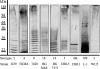
FIG. 1.
Immunoblot analysis of cell wall fractions of S. pneumoniae strains of different capsular serotypes. Cell wall-associated capsule was fractionated by SDS-8% PAGE and, after transfer to nitrocellulose, was reacted with polyclonal antiserum specific to each serotype, as indicated at the bottom of the figure. Protein molecular mass standards (in kilodaltons) were used to standardize each gel to run length and do not indicate actual polymer sizes.
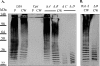
FIG. 2.
Immunoblot analysis of type 2 derivatives. (A) Protoplast-associated (P) or cell wall-associated (CW) capsule from D39, AM1000 (Cps−), KA1501 (Δ_A_), MB526 (Δ_B_), MB516 (Δ_C_), or MB512 (Δ_D_) was separated by SDS-8% PAGE and analyzed by immunoblotting using polyclonal antiserum against type 2 capsule. AM1000, KA1501, MB512, and MB516 contain fourfold more sample than D39 and MB526. Identical results were obtained for two independent isolates of each mutant. (B) Cell wall-associated (CW) capsule of the Rx1-type 2 derivative MB532 (Rx1-2) and its cps2B deletion mutant MB533 (Δ_B_) separated by SDS-10% PAGE and analyzed by immunoblotting using polyclonal antiserum against type 2 capsule. Identical results were obtained with the independently derived Rx1-Δ_cps2B_ mutant MB536 (data not shown).
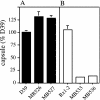
FIG. 3.
Capsule production by Cps2B mutants. Capsule amounts were determined by indirect ELISAs and are expressed relative to D39. (A) D39 and its cps2B deletion derivatives MB526 and MB527. (B) The Rx1-type 2 derivative MB532 (Rx1-2) and its cps2B deletion mutants MB533 and MB536. The P values obtained by comparison to D39 (n = 6) were <0.05 for MB526 (n = 6), <0.005 for MB527 (n = 5), and <0.0005 for MB533 (n = 4) and MB536 (n = 4). MB532 (n = 4) was not different from D39.
FIG. 4.
Comparison of Cps2D protein and tyrosine phosphorylation levels in type 2 capsule mutants. In the upper panel, tyrosine phosphorylation of Cps2D (Cps2D∼P) was detected by using Western immunoblotting with a mouse monoclonal antibody against phosphotyrosine clone PT-66 conjugated to horseradish peroxidase. In the lower panel, Cps2D was detected by using Western immunoblotting and a CpsD-specific polyclonal antiserum. This antiserum recognizes both phosphorylated and nonphosphorylated forms of Cps2D (62), accounting for the doublet seen in the Cps2D blot. For both blots, the Cps− (AM1000), Δ_C_, and Δ_D_ lanes contained threefold more sample than the D39, Δ_A_, Δ_B_, Rx1-2, and Rx1-2 Δ_B_ lanes. The 22- to 32-kDa size range is shown. Comparable results were obtained for two independent isolates of each mutant. Densitometry using ImageJ software (
) was used to determine the intensity of each band. The ratios of the Cps2D∼P and Cps2D intensities for each mutant were normalized to that for D39 or Rx1-2 to obtain the percentage of parent value (% parent value) shown.
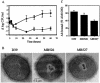
FIG. 5.
Analysis of Cps2B mutants of D39. (A) Blood clearance following i.v. inoculation. The numbers of bacteria were significantly different (P ≤ 0.005) at all time points after 10 h. At 84 h, the mice inoculated with the Δ_cps2B_ mutants had no bacteria remaining, and three of the mice inoculated with D39 had died. ▪, D39 (n = 7); ⧫, Δ_cps2B_ mutants (data combined for MB526 and MB527; n = 10). (B) Electron micrographs. The extracellular substance surrounding D39 and the Cps2B mutants was not present on a nonencapsulated derivative (not shown). Samples were viewed at a magnification of ×50,000. (C) Binding of polyclonal antiserum reactive with S. pneumoniae surface antigens. Results are expressed relative to the value for nonencapsulated AM1000, which is taken as 100%. Reductions in binding occur as a result of blocking of the surface by the capsule. For each strain, n = 3. MB527 was significantly different from D39 (P < 0.05).
Similar articles
- CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae.
Bender MH, Yother J. Bender MH, et al. J Biol Chem. 2001 Dec 21;276(51):47966-74. doi: 10.1074/jbc.M105448200. Epub 2001 Oct 17. J Biol Chem. 2001. PMID: 11606571 - Streptococcus pneumoniae phosphotyrosine phosphatase CpsB and alterations in capsule production resulting from changes in oxygen availability.
Geno KA, Hauser JR, Gupta K, Yother J. Geno KA, et al. J Bacteriol. 2014 Jun;196(11):1992-2003. doi: 10.1128/JB.01545-14. Epub 2014 Mar 21. J Bacteriol. 2014. PMID: 24659769 Free PMC article. - Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in streptococcus pneumoniae.
Morona JK, Paton JC, Miller DC, Morona R. Morona JK, et al. Mol Microbiol. 2000 Mar;35(6):1431-42. doi: 10.1046/j.1365-2958.2000.01808.x. Mol Microbiol. 2000. PMID: 10760144 - Capsules of Streptococcus pneumoniae and other bacteria: paradigms for polysaccharide biosynthesis and regulation.
Yother J. Yother J. Annu Rev Microbiol. 2011;65:563-81. doi: 10.1146/annurev.micro.62.081307.162944. Annu Rev Microbiol. 2011. PMID: 21721938 Review. - Functional organization of the gene cluster involved in the synthesis of the pneumococcal capsule.
García E, Llull D, López R. García E, et al. Int Microbiol. 1999 Sep;2(3):169-76. Int Microbiol. 1999. PMID: 10943410 Review.
Cited by
- Modification of the CpsA protein reveals a role in alteration of the Streptococcus agalactiae cell envelope.
Rowe HM, Hanson BR, Runft DL, Lin Q, Firestine SM, Neely MN. Rowe HM, et al. Infect Immun. 2015 Apr;83(4):1497-506. doi: 10.1128/IAI.02656-14. Epub 2015 Feb 2. Infect Immun. 2015. PMID: 25644003 Free PMC article. - A tyrosine phosphorylation switch controls the interaction between the transmembrane modulator protein Wzd and the tyrosine kinase Wze of Lactobacillus rhamnosus.
Kang HJ, Gilbert C, Badeaux F, Atlan D, LaPointe G. Kang HJ, et al. BMC Microbiol. 2015 Feb 21;15:40. doi: 10.1186/s12866-015-0371-2. BMC Microbiol. 2015. PMID: 25885688 Free PMC article. - Sepsis: mechanisms of bacterial injury to the patient.
Minasyan H. Minasyan H. Scand J Trauma Resusc Emerg Med. 2019 Feb 14;27(1):19. doi: 10.1186/s13049-019-0596-4. Scand J Trauma Resusc Emerg Med. 2019. PMID: 30764843 Free PMC article. Review. - Coordination of capsule assembly and cell wall biosynthesis in Staphylococcus aureus.
Rausch M, Deisinger JP, Ulm H, Müller A, Li W, Hardt P, Wang X, Li X, Sylvester M, Engeser M, Vollmer W, Müller CE, Sahl HG, Lee JC, Schneider T. Rausch M, et al. Nat Commun. 2019 Mar 29;10(1):1404. doi: 10.1038/s41467-019-09356-x. Nat Commun. 2019. PMID: 30926919 Free PMC article. - A tyrosine phosphoregulatory system controls exopolysaccharide biosynthesis and biofilm formation in Vibrio cholerae.
Schwechheimer C, Hebert K, Tripathi S, Singh PK, Floyd KA, Brown ER, Porcella ME, Osorio J, Kiblen JTM, Pagliai FA, Drescher K, Rubin SM, Yildiz FH. Schwechheimer C, et al. PLoS Pathog. 2020 Aug 25;16(8):e1008745. doi: 10.1371/journal.ppat.1008745. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32841296 Free PMC article.
References
- Ambrose, K. D. 2001. Global effects of alterations in capsule production in Streptococcus pneumoniae. University of Alabama at Birmingham, Birmingham.
- Arrecubieta, C., E. Garcia, and R. Lopez. 1995. Sequence and transcriptional analysis of a DNA region involved in the production of capsular polysaccharide in Streptococcus pneumoniae type 3. Gene 167:1-7. - PubMed
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology, vol. 1. John Wiley and Sons, Inc., Boston, Mass.
Publication types
MeSH terms
Substances
Grants and funding
- AI28457/AI/NIAID NIH HHS/United States
- T32 HL007553/HL/NHLBI NIH HHS/United States
- R01 GM053017/GM/NIGMS NIH HHS/United States
- T32 HL07553/HL/NHLBI NIH HHS/United States
- R01 AI028457/AI/NIAID NIH HHS/United States
- GM53017/GM/NIGMS NIH HHS/United States
- T32 GM08111/GM/NIGMS NIH HHS/United States
- T32 GM008111/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous