Telomerase RNA accumulates in Cajal bodies in human cancer cells - PubMed (original) (raw)
Telomerase RNA accumulates in Cajal bodies in human cancer cells
Yusheng Zhu et al. Mol Biol Cell. 2004 Jan.
Abstract
Telomerase synthesizes telomeric DNA repeats at the ends of eukaryotic chromosomes. The RNA component of the enzyme (hTR) provides the template for telomere synthesis, which is catalyzed by telomerase reverse transcriptase (hTERT). Little is known regarding the subcellular localization of hTR and hTERT and the pathway by which telomerase is assembled. Here we report the first glimpse of the detailed subcellular localization of endogenous hTR in human cells, which we obtained by fluorescence in situ hybridization (FISH). Our studies have revealed a distinctive hTR localization pattern in cancer cells. We have found that hTR accumulates within intranuclear foci called Cajal bodies in all typical tumor-derived cell lines examined (in which telomerase is active), but not in primary or ALT cells (where little or no hTERT is present). Accumulation of hTR in the Cajal bodies of primary cells is induced when hTERT is ectopically expressed. Moreover, we report that hTERT is also found in Cajal bodies. Our data suggest that Cajal bodies are involved in the assembly and/or function of human telomerase.
Figures
Figure 1.
Human telomerase RNA is present in intranuclear foci. (A) The predicted secondary structure of hTR is shown (adapted from Chen et al., 2000). Black bars denote regions of complementarity for each hTR probe. Probe 1 is complementary to hTR nts 43–96; probe 2 is complementary to nts 128–183. (B) Fluorescence in situ hybridization (FISH) of HeLa cells was performed with probe 1 (top), probe 2 (middle), or both (bottom). The combination of probes 1 and 2 is used in all subsequent figures except where noted. Fluorescence (hTR) and differential interference contrast (DIC) microscopy images are shown in this and subsequent figures. (C) Top panel shows FISH of HeLa cells performed with a sense probe. hTR FISH signal is lost upon treatment of HeLa cells with RNase A before FISH (middle). There is no detectable accumulation of hTR in VA13 cells, which do not express hTR (bottom).
Figure 2.
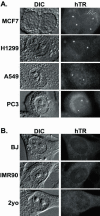
hTR accumulates in intranuclear foci in telomerase-positive cancer cells, but not telomerase-negative primary cells. (A) FISH analysis of hTR localization in the following telomerase-positive cancer cells is shown: MCF7 (breast carcinoma), H1299 (non-small cell lung carcinoma), A549 (lung carcinoma), and PC3 (prostate carcinoma). (B) FISH analysis of hTR localization in the following telomerase-negative primary cells is shown: BJ and IMR90 fibroblasts and 2yo smooth muscle cells.
Figure 4.
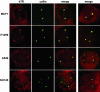
hTR is found in Cajal bodies in all telomerase-positive cancer cell lines examined. hTR FISH (red) was combined with IF with anticoilin antibodies (green) in MCF7, H1299, A549, and DU145 cells. Merged data from an additional cell is shown in the last column.
Figure 3.
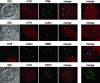
hTR localizes to Cajal bodies. hTR FISH (red) was combined with indirect immunofluorescence (IF) using antibodies against the marker proteins for PML bodies (first row, PML, green) or Cajal bodies (second row, coilin, green) in HeLa cells. Merge panels show merged FISH and IF data; yellow indicates overlap of red and green signals. Merged data from an additional cell is shown in the last column. In the third row, hTR FISH (red) was combined with IF with both anticoilin (green) and anti-SMN antibodies (blue) to visualize Cajal bodies and gems in HeLa-PV cells. Second merge shows the analysis of HeLa-KN cells. In the fourth row, hTR FISH (red) was combined with IF with anti-TRF2 antibodies (green) to visualize telomeres in HeLa cells.
Figure 5.
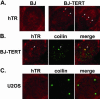
Ectopic expression of hTERT induces accumulation of hTR in nuclear foci including Cajal bodies. (A) hTR was examined by FISH (red) in BJ and BJ-TERT (BJ cells that stably expresses hTERT) cells. Arrows indicate obvious foci of hTR accumulation. (B) hTR FISH (red) was combined with IF with anticoilin antibodies (green) in BJ-TERT cells. Merge panel shows merged FISH and IF data. Arrow indicates a prominent Cajal body in all three panels. (C) hTR FISH (red) was combined with IF with anticoilin antibodies (green) in hTERT- and telomerase-negative U2OS cells. Merge panel shows merged FISH and IF data.
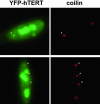
Figure 6.
hTERT is present in Cajal bodies. YFP-TERT was transiently expressed in HeLa cells. Before fixation, cells were preextracted to remove soluble nucleoplasmic YFP-TERT. YFP-TERT (green) localizes to nucleoli and Cajal bodies (red, coilin, indicated in both panels by arrows).
Similar articles
- Visualization of Human Telomerase Localization by Fluorescence Microscopy Techniques.
Abreu E, Terns RM, Terns MP. Abreu E, et al. Methods Mol Biol. 2017;1587:113-125. doi: 10.1007/978-1-4939-6892-3_11. Methods Mol Biol. 2017. PMID: 28324503 - Telomerase reverse transcriptase is required for the localization of telomerase RNA to cajal bodies and telomeres in human cancer cells.
Tomlinson RL, Abreu EB, Ziegler T, Ly H, Counter CM, Terns RM, Terns MP. Tomlinson RL, et al. Mol Biol Cell. 2008 Sep;19(9):3793-800. doi: 10.1091/mbc.e08-02-0184. Epub 2008 Jun 18. Mol Biol Cell. 2008. PMID: 18562689 Free PMC article. - Human telomerase RNA and box H/ACA scaRNAs share a common Cajal body-specific localization signal.
Jády BE, Bertrand E, Kiss T. Jády BE, et al. J Cell Biol. 2004 Mar 1;164(5):647-52. doi: 10.1083/jcb.200310138. Epub 2004 Feb 23. J Cell Biol. 2004. PMID: 14981093 Free PMC article. - Expression of telomerase genes in thyroid carcinoma.
Hoang-Vu C, Boltze C, Gimm O, Poremba C, Dockhorn-Dworniczak B, Köhrle J, Rath FW, Dralle H. Hoang-Vu C, et al. Int J Oncol. 2002 Aug;21(2):265-72. Int J Oncol. 2002. PMID: 12118320 Review. - Telomerase redefined: integrated regulation of hTR and hTERT for telomere maintenance and telomerase activity.
Cairney CJ, Keith WN. Cairney CJ, et al. Biochimie. 2008 Jan;90(1):13-23. doi: 10.1016/j.biochi.2007.07.025. Epub 2007 Aug 6. Biochimie. 2008. PMID: 17854971 Review.
Cited by
- Composition and Function of Telomerase-A Polymerase Associated with the Origin of Eukaryotes.
Schrumpfová PP, Fajkus J. Schrumpfová PP, et al. Biomolecules. 2020 Oct 8;10(10):1425. doi: 10.3390/biom10101425. Biomolecules. 2020. PMID: 33050064 Free PMC article. Review. - Trimethylguanosine capping selectively promotes expression of Rev-dependent HIV-1 RNAs.
Yedavalli VS, Jeang KT. Yedavalli VS, et al. Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14787-92. doi: 10.1073/pnas.1009490107. Epub 2010 Aug 2. Proc Natl Acad Sci U S A. 2010. PMID: 20679221 Free PMC article. - Loss of Human TGS1 Hypermethylase Promotes Increased Telomerase RNA and Telomere Elongation.
Chen L, Roake CM, Galati A, Bavasso F, Micheli E, Saggio I, Schoeftner S, Cacchione S, Gatti M, Artandi SE, Raffa GD. Chen L, et al. Cell Rep. 2020 Feb 4;30(5):1358-1372.e5. doi: 10.1016/j.celrep.2020.01.004. Cell Rep. 2020. PMID: 32023455 Free PMC article. - The role of the plant nucleolus in pre-mRNA processing.
Brown JW, Shaw PJ. Brown JW, et al. Curr Top Microbiol Immunol. 2008;326:291-311. doi: 10.1007/978-3-540-76776-3_16. Curr Top Microbiol Immunol. 2008. PMID: 18630759 Free PMC article. Review. - Radiolabeled Oligonucleotides Targeting the RNA Subunit of Telomerase Inhibit Telomerase and Induce DNA Damage in Telomerase-Positive Cancer Cells.
Jackson MR, Bavelaar BM, Waghorn PA, Gill MR, El-Sagheer AH, Brown T, Tarsounas M, Vallis KA. Jackson MR, et al. Cancer Res. 2019 Sep 15;79(18):4627-4637. doi: 10.1158/0008-5472.CAN-18-3594. Epub 2019 Jul 16. Cancer Res. 2019. PMID: 31311806 Free PMC article.
References
- Avilion, A.A., Piatyszek, M.A., Gupta, J., Shay, J.W., Bacchetti, S., and Greider, C.W. (1996). Human telomerase RNA and telomerase activity in immortal cell lines and tumor tissues. Cancer Res. 56, 645–650 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources