Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment - PubMed (original) (raw)
Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment
Andrei I Ivanov et al. Mol Biol Cell. 2004 Jan.
Abstract
The adherens junction (AJ) and tight junction (TJ) are key regulators of epithelial polarity and barrier function. Loss of epithelial phenotype is accompanied by endocytosis of AJs and TJs via unknown mechanisms. Using a model of calcium depletion, we defined the pathway of internalization of AJ and TJ proteins (E-cadherin, p120 and beta-catenins, occludin, JAM-1, claudins 1 and 4, and ZO-1) in T84 epithelial cells. Proteinase protection assay and immunocytochemistry revealed orchestrated internalization of AJs and TJs into a subapical cytoplasmic compartment. Disruption of caveolae/lipid rafts did not prevent endocytosis, nor did caveolin-1 colocalize with internalized junctional proteins. Furthermore, AJ and TJ proteins did not colocalize with the macropinocytosis marker dextran. Inhibitors of clathrin-mediated endocytosis blocked internalization of AJs and TJs, and junctional proteins colocalized with clathrin and alpha-adaptin. AJ and TJ proteins were observed to enter early endosomes followed by movement to organelles that stained with syntaxin-4 but not with markers of late and recycling endosomes, lysosomes, or Golgi. These results indicate that endocytosis of junctional proteins is a clathrin-mediated process leading into a unique storage compartment. Such mechanisms may mediate the disruption of intercellular contacts during normal tissue remodeling and in pathology.
Figures
Figure 1.
Depletion of extracellular calcium decreases proteolytic sensitivity of AJ and TJ proteins by inducing their translocation into a subapical intracellular compartment. Confluent T84 monolayers were incubated in either complete T84 medium or in calcium-free S-MEM for 120 min followed by brief exposure to (A) trypsin (0.05%) or (B) α-chymotrypsin (α-CT; 0.5%) solutions as described in MATERIALS AND METHODS. Thereafter, cells were lysed and amounts of junctional proteins in total cell lysates determined by Western blotting. In Panel C, another subset of control and calcium-depleted T84 monolayers was fixed, double-labeled with actin and E-cadherin, β-catenin, JAM-1, or ZO-1, and analyzed by confocal microscopy (x-z images). Calcium depletion decreases the sensitivity of E-cadherin, JAM-1, and occludin to proteolysis indicating internalization of these proteins. Reconstructed confocal images in the x-z plane reveal internalization of E-cadherin, β-catenin, JAM-1, and ZO-1 into a subapical cytosolic compartment located under the apical F-actin (arrows).
Figure 2.
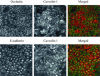
Calcium depletion induces rapid orchestrated endocytosis of AJ and TJ proteins. Confluent T84 monolayers were incubated in S-MEM for indicated times and intracellular localization of AJ proteins E-cadherin, β-catenin, and TJ proteins occludin, JAM-1 was determined by immunofluorescence labeling and confocal microscopy. In control T84 cells, all junctional proteins are localized at intercellular contacts revealing a characteristic “chicken wire” staining pattern. Depletion of extracellular calcium leads to a rapid orchestrated translocation of all junctional proteins from the cell border into centrally located ring-like structures.
Figure 3.
Internalized AJ and TJ proteins occupy different parts of a subapical endosomal compartment. T84 cells were incubated for 60 min in S-MEM followed by double immunolabeling for ZO-1 (green) with other junctional proteins (red). In the ring-like structures, ZO-1 colocalizes (yellow) with TJ proteins occludin, JAM-1, and claudin-4 (arrows), but not with AJ proteins E-cadherin, β-catenin, and p120 catenin.
Figure 4.
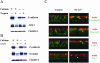
Inhibition of caveolar/lipid raft-mediated endocytosis does not block internalization of junctional proteins in calciumdepleted cells. T84 cells were preincubated for 60 min in serum-free T84 medium with either cholesterol oxidase (2 units/ml) or MβCD (10 mM) followed by a 120-min incubation in serum-free S-MEM containing the same concentrations of inhibitors. Control monolayers were incubated for the indicated times in serum-free T84 medium and S-MEM. Localization of E-cadherin and occludin was determined by immunofluorescence labeling and confocal microscopy. The bottom panel is a positive control, an effect of the inhibitors on cholera toxin uptake. As can be seen, both inhibitors attenuate cholera toxin uptake but fail to prevent internalization of junctional proteins.
Figure 5.
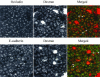
Internalized AJ and TJ proteins do not colocalize with the caveolar marker caveolin-1. T84 cells were depleted of calcium for 15 min and double-immunolabeled for caveolin-1 (red) and E-cadherin or occludin (green). As can be seen, caveolin-1 labeling does not show characteristic ring-like structures and does not colocalize with E-cadherin or occludin. Occasional yellow color observed in the lower merged image is due to focal oversaturation of the caveolin-1 signal.
Figure 6.
Differential effects of macropinocytosis inhibitors on internalization of junctional proteins in calcium-depleted cells. T84 cells were preincubated for 60 min in regular T84 medium with either 100 μM of inhibitor of the Na+/H+ exchanger, EIPA or 50 μM of the PI 3-kinase inhibitor LY 294002 followed by a 120-min incubation in S-MEM containing the same concentrations of inhibitors. Control monolayers were incubated in T84 medium and S-MEM containing vehicle (0.1% DMSO). Localization of E-cadherin and occludin was determined by immunofluorescence labeling and confocal microscopy. The effect of these inhibitors on internalization of fluorescently labeled dextran, was used as a positive control (bottom panel). Both types of macropinocytosis inhibitors prevent dextran uptake, however one of them (EIPA) significantly attenuates the internalization of junctional proteins, whereas another (LY 294002) is ineffective.
Figure 7.
Internalized AJ and TJ proteins do not colocalize with the macropinocytosis marker, dextran. T84 cells were incubated for 60 min in S-MEM containing a 1 mg/ml fluorescently labeled dextran (red) followed by immunostaining for E-cadherin or occludin (green). Although internalized dextran localizes in an intracellular compartment resembling that of E-cadherin and occludin, it does not colocalize (arrows), suggesting a distinct endosomal population.
Figure 8.
Inhibition of clathrin-mediated endocytosis blocks internalization of junctional proteins in calcium-depleted cells. T84 cells were preincubated for 60 min in either hypertonic (0.4 M sucrose) or acidic (pH 5.5) T84 media or with POA (20 μM) followed by a 120-min incubation in hypertonic or acidic or POA-containing S-MEM. Control monolayers were incubated in complete T84 medium and S-MEM. Localization of E-cadherin and occludin was determined by immunofluorescence labeling and confocal microscopy. All three inhibitors, which are known to prevent the formation of clathrin-coated pits, effectively block internalization of junctional proteins.
Figure 9.
Internalized AJ and TJ proteins colocalize with a marker of clathrin-mediated endocytosis. T84 cells were depleted of calcium for 15 min and double-immunolabeled for occludin and E-cadherin (green) with α-adaptin (red). As can be seen, in the ring-like structures (arrows) occludin and Ecadherin clearly colocalize with the marker of clathrin-mediated endocytosis.
Figure 10.
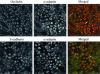
Internalized AJ and TJ proteins transiently localize in early endosomes. Intact T84 cell monolayers or monolayers depleted of calcium for 15 and 60 min were double-immunolabeled for occludin (green) and early endosomal marker, Rab5 (red). As can be seen, internalized occludin colocalizes with the early endosomal markers at early (5 min; arrows) but not late (60 min) times of endocytosis.
Figure 11.
Internalized AJ and TJ proteins are delivered into different endosomal populations within a storage compartment for basolateral proteins. T84 cells were depleted of calcium for 60 min and double-immunolabeled for E-cadherin and occludin with markers of the storage compartment for basolateral proteins, syntaxin-4 and Na+/K+ ATPase. As can be seen, both internalized E-cadherin and occludin colocalize with syntaxin-4 (arrows) but only E-cadherin colocalize with Na+/K+ ATPase (asterisks).
Similar articles
- The epithelium in inflammatory bowel disease: potential role of endocytosis of junctional proteins in barrier disruption.
Ivanov AI, Nusrat A, Parkos CA. Ivanov AI, et al. Novartis Found Symp. 2004;263:115-24; discussion 124-32, 211-8. Novartis Found Symp. 2004. PMID: 15669638 Review. - Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function.
Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Hopkins AM, et al. J Cell Sci. 2003 Feb 15;116(Pt 4):725-42. doi: 10.1242/jcs.00300. J Cell Sci. 2003. PMID: 12538773 - A membrane fusion protein αSNAP is a novel regulator of epithelial apical junctions.
Naydenov NG, Brown B, Harris G, Dohn MR, Morales VM, Baranwal S, Reynolds AB, Ivanov AI. Naydenov NG, et al. PLoS One. 2012;7(4):e34320. doi: 10.1371/journal.pone.0034320. Epub 2012 Apr 2. PLoS One. 2012. PMID: 22485163 Free PMC article. - Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins.
Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Kucharzik T, et al. Am J Pathol. 2001 Dec;159(6):2001-9. doi: 10.1016/S0002-9440(10)63051-9. Am J Pathol. 2001. PMID: 11733350 Free PMC article. - Endocytosis and recycling of tight junction proteins in inflammation.
Utech M, Mennigen R, Bruewer M. Utech M, et al. J Biomed Biotechnol. 2010;2010:484987. doi: 10.1155/2010/484987. J Biomed Biotechnol. 2010. PMID: 20011071 Free PMC article. Review.
Cited by
- A Quantity-Dependent Nonlinear Model of Sodium Cromoglycate Suppression on Beta-Conglycinin Transport.
Zheng Z, Han J, Chen X, Zheng S. Zheng Z, et al. Int J Mol Sci. 2024 Jun 17;25(12):6636. doi: 10.3390/ijms25126636. Int J Mol Sci. 2024. PMID: 38928351 Free PMC article. - PKCι interacts with Rab14 and modulates epithelial barrier function through regulation of claudin-2 levels.
Lu R, Dalgalan D, Mandell EK, Parker SS, Ghosh S, Wilson JM. Lu R, et al. Mol Biol Cell. 2015 Apr 15;26(8):1523-31. doi: 10.1091/mbc.E14-12-1613. Epub 2015 Feb 18. Mol Biol Cell. 2015. PMID: 25694446 Free PMC article. - Type I gamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin.
Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA. Ling K, et al. J Cell Biol. 2007 Jan 29;176(3):343-53. doi: 10.1083/jcb.200606023. J Cell Biol. 2007. PMID: 17261850 Free PMC article. - Microtubules regulate disassembly of epithelial apical junctions.
Ivanov AI, McCall IC, Babbin B, Samarin SN, Nusrat A, Parkos CA. Ivanov AI, et al. BMC Cell Biol. 2006 Mar 1;7:12. doi: 10.1186/1471-2121-7-12. BMC Cell Biol. 2006. PMID: 16509970 Free PMC article. - Cancer stem-like cells evade CD8+CD103+ tumor-resident memory T (TRM) lymphocytes by initiating an epithelial-to-mesenchymal transition program in a human lung tumor model.
Corgnac S, Damei I, Gros G, Caidi A, Terry S, Chouaib S, Deloger M, Mami-Chouaib F. Corgnac S, et al. J Immunother Cancer. 2022 Apr;10(4):e004527. doi: 10.1136/jitc-2022-004527. J Immunother Cancer. 2022. PMID: 35418483 Free PMC article.
References
- Ameen, N.A., and Salas, P.J.I. (2000). Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic 1, 76–83. - PubMed
- Amyere, M., Mettlen, M., Van Der Smissen, P., Platek, A., Payrastre, B., Veithen, A., and Courtoy, P.J. (2002). Origin, originality, functions, subversions and molecular signaling of macropinocytosis. Int. J. Med. Microbiol. 291, 487–494. - PubMed
- Basuroy, S., Sheth, P., Kuppuswamy, D., Balasubramanian, S., Ray, R.M., and Ray, R.K. (2003). Expression of kinase-inactive s-Src delays oxidative stressinduced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J. Biol. Chem. 278, 11916–11924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R29 DK055679/DK/NIDDK NIH HHS/United States
- DK 55679/DK/NIDDK NIH HHS/United States
- DK 61379/DK/NIDDK NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- R01 DK055679/DK/NIDDK NIH HHS/United States
- DK 59888/DK/NIDDK NIH HHS/United States
- DK 64399/DK/NIDDK NIH HHS/United States
- R01 DK061379/DK/NIDDK NIH HHS/United States
- R01 DK059888/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous