Formation of facultative heterochromatin in the absence of HP1 - PubMed (original) (raw)
Formation of facultative heterochromatin in the absence of HP1
Nick Gilbert et al. EMBO J. 2003.
Abstract
Facultative heterochromatin is a cytological manifestation of epigenetic mechanisms that regulate gene expression. Constitutive heterochromatin is marked by distinctive histone H3 methylation and the presence of HP1 proteins, but the chromatin modifications of facultative heterochromatin are less clear. We have examined histone modifications and HP1 in the facultative heterochromatin of nucleated erythrocytes and show that mouse and chicken erythrocytes have different mechanisms of heterochromatin formation. Mouse embryonic erythrocytes have abundant HP1, increased tri-methylation of H3 at K9 and loss of H3 tri-methylation at K27. In contrast, we show that HP1 proteins are lost during the differentiation of chicken erythrocytes, and that H3 tri-methylation at both K9 and K27 is reduced. This coincides with the appearance of the variant linker histone H5. HP1s are also absent from erythrocytes of Xenopus and zebrafish. Our data show that in the same cell lineage there are different mechanisms for forming facultative heterochromatin in vertebrates. To our knowledge, this is the first report of cell types that lack HP1s and that have gross changes in the levels of histone modifications.
Figures
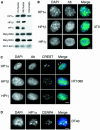
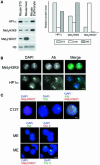
Fig. 1. Distribution of HP1 isoforms in human, mouse and chicken cells. (A) Immunoblotting of proteins isolated from whole human (hu) or mouse (mo) nuclei, or from mouse chromatin using antisera that detect HP1s α, β and γ, di-methyl H3-K9 (met2H3-K9), and di-methyl H3-K4 (met2H3-K4). An antibody that recognizes H3 is used as a loading control. (B) Immunofluorescence of mouse 3T3 nuclei fixed with pFa using antibodies that detect HP1s α, β and γ (green). DNA was counterstained with DAPI (blue) to highlight the foci of heterochromatin. (C) Co-immunostaining of human HT1080 cells using antibodies that detect HP1s α, β and γ (green) and with CREST antiserum, which detects centromeric antigens (red). DNA was counterstained with DAPI (blue). Arrowheads indicate where foci of HP1α and CREST staining are coincident. (D) Co-immunostaining of chicken DT40 cells with antibody recognizing HP1α (green) and CENP-A (red). Scale bars, 5 µm.
Fig. 2. Distribution of metH3-K9 in human, mouse and chicken cells. (A) Immunostaining of mouse 3T3 with antisera (green) raised against a linear peptide dimethylated at K9 of H3 (met2H3-K9 linear; Boggs et al., 2002), or against a branched di-methylated peptide (met3H3-K9 branched, but which detects tri-methylated H3-K9; Peters et al., 2001) or against a linear peptide tri-methylated at K27 (Silva et al., 2003). DNA was counterstained with DAPI (blue). (B) Co-immunostaining of human HT1080 cells with metH3-K9 linear and branched antibodies (green) and CREST serum (red). DNA was counterstained with DAPI (blue). Arrows indicate sites of co-staining. Arrow heads indicate foci of met3H3-K9 that do not correspond to centromeres detected by CREST. (C) Co-localization of HP1α with tri-methyl H3-K9 in mouse 3T3, human HT1080 and chicken DT40 cells. Scale bars, 5 µm.
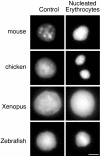
Fig. 3. Chromatin condensation in vertebrate nucleated erythrocytes. DAPI staining of mouse, chicken, Xenopus and zebrafish nucleated erythrocytes (right hand panel) contrasted with the staining pattern of nuclei from control tissues (mouse, Xenopus and zebrafish liver) and chicken DT40 cells. Scale bar, 5 µm.
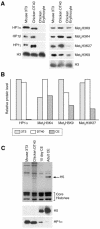
Fig. 4. Loss of HP1s from chicken erythrocytes. (A) Western blot of mouse 3T3, chicken DT40 and adult chicken erythrocyte nuclear proteins with antibodies detecting HP1 isoforms, di- (met2H3-K9) and tri- (met3H3-K9) methylated H3 K9, and H3 methylated at K4 and K27. Antibody detecting H3 is used as a control. (B) Quantification of HP1α, met2H3-K4, met3H3-K9 and met3H3-K27 levels in mouse, DT40 and chicken erythrocyte nuclei. Signals were normalized with respect to total histone H3. (C) Coomassie-stained gel of mouse 3T3, chicken DT40, chicken day 10 embryonic erythrocytes and adult chicken erythrocytes. Histone H5 is indicated by an arrow. Western blots of equivalent samples probed with antibodies against H5 and HP1α are shown below.
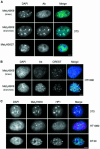
Fig. 5. Absence of HP1α from chicken, fish and Xenopus erythrocytes. (A) Western blot of proteins from fish liver and nucleated erythrocytes, and from Xenopus tail bud tadpole nuclei and erythrocytes, using antibodies raised against Xenopus HP1α and HP1γ. HP1β isoforms could not be detected in Xenopus or zebrafish cells using available antibodies. (B) Immunostaining of chicken and Xenopus nuclei with met3H3-K9 or met3H3-K27 antisera, or HP1α antibody (green) and DNA (blue). Scale bar, 5 µm.
Fig. 6. HP1α and methylated H3 in mouse erythrocytes. (A) Western blot of proteins from mouse 3T3, liver and embryonic erythrocyte nuclei using antibodies detecting HP1α, met3H3-K9 and met3H3-K27. H3 antibody is used as a loading control. The graph to the right shows the quantification of these blots. (B) Immunostaining of HP1α and met3H3-K9 (green) in mouse embryonic nucleated erythrocytes. DNA was counterstained with DAPI (blue). (C) Female C127 cells: immunostaining for met3H3-K27 (red), RNA FISH for Xist (green) and double immunostaining for met3H3-K27 (red) and RNA FISH for Xist (green). Mouse embryonic nucleated erythrocytes: RNA FISH for Xist (green) followed by DNA FISH for X chromosome (red), and immunostaining for met3H3-K27 (red) followed by DNA FISH for X chromosome (green). Scale bar, 5 µm.
Similar articles
- Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin.
Peters AH, Mermoud JE, O'Carroll D, Pagani M, Schweizer D, Brockdorff N, Jenuwein T. Peters AH, et al. Nat Genet. 2002 Jan;30(1):77-80. doi: 10.1038/ng789. Epub 2001 Dec 10. Nat Genet. 2002. PMID: 11740497 - Epigenetic regulation of mammalian pericentric heterochromatin in vivo by HP1.
Kourmouli N, Sun YM, van der Sar S, Singh PB, Brown JP. Kourmouli N, et al. Biochem Biophys Res Commun. 2005 Nov 25;337(3):901-7. doi: 10.1016/j.bbrc.2005.09.132. Epub 2005 Sep 30. Biochem Biophys Res Commun. 2005. PMID: 16213461 - Nuclear levels and patterns of histone H3 modification and HP1 proteins after inhibition of histone deacetylases.
Bártová E, Pacherník J, Harnicarová A, Kovarík A, Kovaríková M, Hofmanová J, Skalníková M, Kozubek M, Kozubek S. Bártová E, et al. J Cell Sci. 2005 Nov 1;118(Pt 21):5035-46. doi: 10.1242/jcs.02621. J Cell Sci. 2005. PMID: 16254244 - How HP1 Post-Translational Modifications Regulate Heterochromatin Formation and Maintenance.
Sales-Gil R, Vagnarelli P. Sales-Gil R, et al. Cells. 2020 Jun 12;9(6):1460. doi: 10.3390/cells9061460. Cells. 2020. PMID: 32545538 Free PMC article. Review. - Linking Heterochromatin Protein 1 (HP1) to cancer progression.
Dialynas GK, Vitalini MW, Wallrath LL. Dialynas GK, et al. Mutat Res. 2008 Dec 1;647(1-2):13-20. doi: 10.1016/j.mrfmmm.2008.09.007. Epub 2008 Sep 24. Mutat Res. 2008. PMID: 18926834 Free PMC article. Review.
Cited by
- High- and low-mobility populations of HP1 in heterochromatin of mammalian cells.
Schmiedeberg L, Weisshart K, Diekmann S, Meyer Zu Hoerste G, Hemmerich P. Schmiedeberg L, et al. Mol Biol Cell. 2004 Jun;15(6):2819-33. doi: 10.1091/mbc.e03-11-0827. Epub 2004 Apr 2. Mol Biol Cell. 2004. PMID: 15064352 Free PMC article. - Analysis of active and inactive X chromosome architecture reveals the independent organization of 30 nm and large-scale chromatin structures.
Naughton C, Sproul D, Hamilton C, Gilbert N. Naughton C, et al. Mol Cell. 2010 Nov 12;40(3):397-409. doi: 10.1016/j.molcel.2010.10.013. Mol Cell. 2010. PMID: 21070966 Free PMC article. - Heterochromatin protein 1gamma epigenetically regulates cell differentiation and exhibits potential as a therapeutic target for various types of cancers.
Takanashi M, Oikawa K, Fujita K, Kudo M, Kinoshita M, Kuroda M. Takanashi M, et al. Am J Pathol. 2009 Jan;174(1):309-16. doi: 10.2353/ajpath.2009.080148. Epub 2008 Dec 4. Am J Pathol. 2009. PMID: 19056850 Free PMC article. - Histone modifications and nuclear architecture: a review.
Bártová E, Krejcí J, Harnicarová A, Galiová G, Kozubek S. Bártová E, et al. J Histochem Cytochem. 2008 Aug;56(8):711-21. doi: 10.1369/jhc.2008.951251. Epub 2008 May 12. J Histochem Cytochem. 2008. PMID: 18474937 Free PMC article. Review. - Mouse centric and pericentric satellite repeats form distinct functional heterochromatin.
Guenatri M, Bailly D, Maison C, Almouzni G. Guenatri M, et al. J Cell Biol. 2004 Aug 16;166(4):493-505. doi: 10.1083/jcb.200403109. Epub 2004 Aug 9. J Cell Biol. 2004. PMID: 15302854 Free PMC article.
References
- Armstrong S.J., Hulten,M.A., Keohane,A.M. and Turner,B.M. (1997) Different strategies of X inactivation in germinal and somatic cells: histone H4 underacetylation does not mark the inactive X chromosome in the mouse germline. Exp. Cell Res., 230, 399–402. - PubMed
- Bannister A.J., Zegerman,P., Partridge,J.F., Miska,E.A., Thomas,J.O., Allshire,R.C. and Kouzarides,T. (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature, 410, 120–124. - PubMed
- Bergman M.G., Wawra,E. and Winge,M. (1988) Chicken histone H5 inhibits transcription and replication when introduced into proliferating cells by microinjection. J. Cell Sci., 91, 201–209. - PubMed
- Boggs B.A., Cheung,P., Heard,E., Spector,D.L., Chinault,A.C. and Allis,C.D. (2002) Differentially methylated forms of histone H3 show unique association patterns with inactive human X chromosomes. Nat. Genet., 30, 73–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources